|
Spectra are produced when electromagnetic radiation is emitted by a source or absorbed from another source. |
|
The visible spectrum
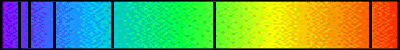
The region of the electromagnetic spectrum that humans are able to see is very limited, called the visible spectrum. We are all familiar with the colours of the visible spectrum:
Red, orange, yellow, green, blue, indigo and violet.
We may not be quite so familiar with the wavelength range covered:
- Red light begins at wavelength 760 nm (1nm = 1 x 10-9 metres)
- Blue light ends the high energy region of the visible spectrum at 360 nm
Use the slider on the animation below to see the relationship between colour, wavelength, frequency and energy of visible light.
The term 'ultra violet' (literally, beyond violet) refers to electromagnetic waves with energy greater than the visible range. The term infra-red refers to wavelengths with lower energy than the visible range.
A visible spectrum is produce when a light source (sunbeam, torch, laser etc) passes through a refracting prism (piece of glass, or a Diffraction grating) and the light is bent through an angle that depends on the wavength of the light passing through.
If the light wavelength is long ( for instance, red light, wavelength 700nm) it is not deviated as much as a short wavelength (e.g. blue light, wavelength 400nm).
Hence, any source of light consisting of several different wavelengths may be separated and displayed on a screen or the different wavelengths may be detected electronically and displayed.
If the light source contains all possible wavelengths (e.g. white light) then a continuous spectrum results (eg a rainbow)
Continuous spectra
A spectrum may be continuous, or may comprise bright lines (an emission spectrum), or dark lines (an absorption spectrum) superimposed on a background.
A continuous spectrum results when the gas pressures are higher, so that lines are broadened by collisions between the atoms until they are smeared into a continuum. We may view a continuum spectrum as an emission spectrum in which the lines overlap with each other and can no longer be distinguished as individual emission lines.
Line spectra
These, as would be imagined, are spectra that appear as specific discrete lines on a background. They may be either emission or absorption.
Emission spectra are produced by passing electrical or thermal energy through gases in which the atoms do not experience many collisions (because of the low density). The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels.
As shown, emission spectra result from electrons within a sample becoming excited and moving to higher energy levels. They cannot stay at the higher level for very long and they fall back to the ground state, releasing energy equivalent to the difference between the energies of the two levels.
Absorption spectra
An absorption spectrum occurs when light of all wavelengths passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies. As the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) superimposed on the continuous spectrum.
This phenomenon is used in Atomic Absorption Spectrophotometry (AAS) for trace analysis of ions in solution.
Summary
|
Continuous, Emission, and Absorption Spectra
|
|
|
|
|
|
|