| Covalent bonding usually occurs between non-metal atoms. It is the simplest kind of chemical bonding. |
|
Atoms are held together in covalent bonding by means of shared pairs of electrons. This constitutes a single covalent bond.
The various theories as to how and why these bonds are formed are discussed below. It is important to recognise that formation of covalent bonds (or any other type of bond) is an exothermic process, one that releases energy. Similarly, to break a bond always requires energy.
Any cleavage (breakage) of covalent bonds involves a chemical reaction and new substances are necessarily formed.
Atoms are unstable unless they have fully occupied outer shells of electrons. We know from observation and inference that atoms bond together to make larger structures.
In order to explain how this happens, different theories are proposed that explain these observations. All of these theories revolve around the accepted idea that opposite charges attract (electrostatic attraction).
|
The scientific method A theory helps to explain all of the observable facts. If it fits all of the evidence, then it is adopted as a useful explanation of how the universe works. However, if further observations throw up anomalies, or observations that cannot be explained by the current theory, then it must be either adapted, or even discarded completely. |
Linear combination of atomic orbitals
This theory of bonding proposes that molecules are formed by overlapping regions of space, allowing atoms to mutually share pairs of electrons. This pair of electrons is then held in a position between the two nuclei of the atoms holding them together. We can consider this theory by looking at the hydrogen molecule.
The hydrogen molecule is the simplest structure formed between atoms. In this case two hydrogen atoms share one pair of electrons between them, forming a diatomic molecule.
The negative charge clouds (atomic orbitals) overlap, placing a region of negative charge between the two hydrogen nuclei.
The electrons in each atom is also attracted to the nucleus of the neighbouring atom. The atoms are pulled together. This view of chemical bonding is known as covalent bonding as the valence electrons of both the hydrogen atoms are shared. ('co' = shared and 'valent' = valence electrons)
The regions of space in which electrons are found are called orbitals and we say that this bond arises from direct overlap of atomic orbitals. This is sometimes called the Linear Combination of Atomic Orbital Theory (LCAO).
This model works very well for a simple picture of bonding between atoms. The nuclei of the atoms are held to one another by the positive - negative - positive attractions caused by electron pairs between the nuclei.
The only atoms that occur in nature not combined into some kind of structure are the inert gases. These all have full outer (valence) shells. It seems to be that this particular electronic configuration is stable for some reason.
| Atom | configuration |
| helium | 2 |
| neon | 2,8 |
| argon | 2,8,8 |
| krypton | 2,8,18,8 |
| xenon | 2,8,18,18,8 |
Covalent bonding occurs between atoms so that they can attain a full outer shell by SHARING electrons.
In the case of hydrogen molecules, above, the atom would have a full outer shell if it were to attain two electrons. By an arrangement in which two atoms share one pair of electrons, each atom achieves the requirement. The system is now stable, it does not change further.
Covalent bonding usually occurs between non-metal atoms; they attain a full outer shell of electrons by sharing electrons. However, as we shall see in the following section there are exceptions.
- Covalent bonding happens when non-metal atoms combine
- Electron pairs are shared to give a full outer shell of valence electrons to each atom
- The final units are molecules of atoms joined together by shared pairs of electrons
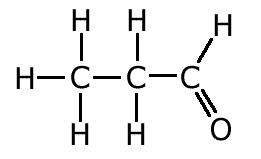
The covalent bond (shared pair) may be represented by a line to show typical stick structures such as:
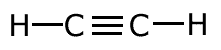 |
 |
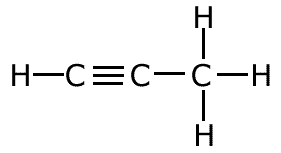 |
|
ethyne
|
propanal
|
propyne
|
A single line is drawn to represent one pair of electrons. A double bond containing two pairs of electrons between atoms is shown as a double line, and three pairs, a triple bond, as a three lines. It is not possible for two atoms to share more than three pairs of electrons.

