|
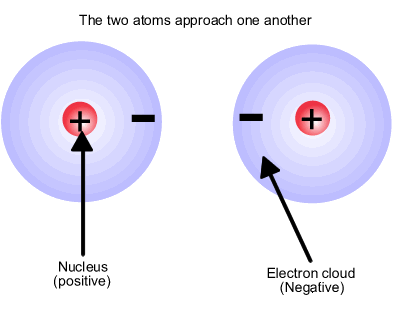
Atoms consist of positive nuclear centres and negative charge clouds, also known as electron domains. The atoms are neutral overall but inherently unstable. This neutrality does not prevent the nucleus also being attracted to electron domains in other atoms. If approaching atoms have suitable regions of space (orbitals) in which to contain the electrons, they can share electron pairs between the two nuclei forming an electrostatic bond, positive nucleus1 to electron domain to positive nucleus2. |
The instability of isolated atoms
Atoms cannot exist on their own. They are highly reactive in this condition.
This is an empirical observation drawn from the fact that the only substances that exist as single atoms are the noble gases.
It can be inferred that the formation of the bulk structures of the elements, compounds and molecules is due to this chemical reactivity
The covalent bond
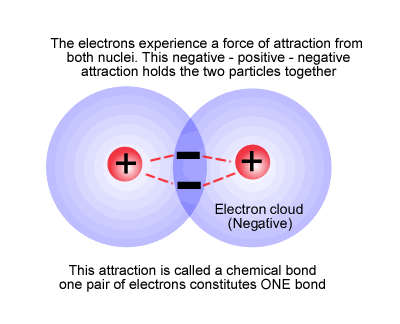
This happens when the electrons from one atom are attracted by the nucleus of a second atom, but not so much as to be removed from the first atom (see electronegativity) and vice versa. The electrons remain in a region of space between the two nuclei, effectively attaching the nuclei together (bond), forming a new species with more than one nuclear centre, a molecule.
The simplest molecule is hydrogen, sometimes called dihydrogen, H2.
The hydrogen molecule
Electronegativity
This will be treated in greater depth in the section on periodicity, but suffice to say that it is the attracting power of an atom for an electron pair along a covalent bond.
In bonding this gives us an idea about whether an atom will share electrons with another or whether it will lose electrons to another atom. The latter case gives rise to ionic bonding.
Electronegativity values range from 0.7 to 4.0 on the Pauling scale (the most often used). If there is a large difference in electronegativity between atoms then covalent bonds do not form. Hence, covalent bonds tend to form between atoms close together in electronegativity.
The general rule is that metals and non-metals form ionic bonds unless they have similar electronegativity values.
Non-metals always bond with other non-metals via covalent bonding.
The nature of covalent bonds
As stated above, the bond consists of a pair of electrons between two nuclear centres holding them together by electrostatic attraction. In the same way that all nuclear centres are not the same, so every bond has its own characteristics in terms of bond length and strength.
The bond length is measured as the distance between the two nuclear centres.
The bond strength is the quantity of energy that must be used to break the two atoms apart. This is also called the bond dissociation enthalpy. It is equal in magnitude (but opposite in sign) to the amount of energy released by the two bonding atoms when forming the bond.
The shorter the bond generally means the stronger the bond, but there are exceptions. For example, fluorine-fluorine bonds are very short, but also unusually weak.
Bonding between non-identical atoms
The shared pair of electrons will not be held equally by the two atoms and this will create an uneven charge distribution, giving rise to polarity. This is treated in section 2.30.

