|
The valence shell is the outer shell of electrons. These are the electrons that are involved in bonding. |
|
In the section on Lewis structures we discussed the idea that electrons tend to exist in pairs around the outer shells of atoms. The stable situation appears to be for the electrons to adopt a situation where four pairs are in the outer (valence) shell (with the exception of hydrogen, which is 'full' with one pair of electrons). This arrangement of eight electrons is called the 'octet rule'.
|
Methane, CH4, molecule showing the four pairs of electrons around the central carbon atom. 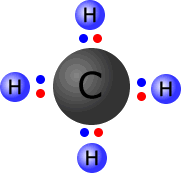
|
Like charges repel, negative charges repel other negative charges. The electrons are constrained around the nucleus in pairs. Each pair exerts a repulsion on other electron pairs. To ascertain the actual shape adopted by molecules we must consider these repulsions.
The electrons in a domain are also attracted to the nucleus. Thus any repulsion will cause the electron pairs to move as far apart as possible, while still remaining attached to the central nucleus.
It is rather like having two magnetic north poles attached to two strings with the strings attached to a central pivot. The magnets will push as far away as possible while staying tied to the central pivot.
The final electronic shape adopted depends on the number of electron domains around the nucleus.
The two domains are repelled as far apart as possible while remaining fixed to the nucleus. They adopt an arrangement in which they are at 180º to one another.
|
Here we can see the beryllium dichloride molecule that has only two pairs of electrons on the central atom. They move as far apart as possible and adopt an arrangement as shown. 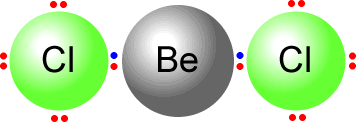 |
Three electron domains move as far apart as possible while remaining attached to the central atom. They adopt a trigonal planar arrangement in which the angle formed between the electron pairs is 120º.
|
Here we can see the boron trifluoride molecule. 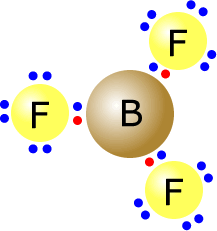
It has three pairs of electrons on the central atom. They move as far apart as possible and adopt an arrangement as shown. The bond angle subtended by the F-B-F atoms is 120º in all cases. |
Four electron domains adopt a tetrahedral arrangement. This is a three dimensional shape that can be considered formed from the peaks of a
with the central atom in the very centre of the pyramid. The molecule methane has electron domains around the central atom, which adopt a tetrahedral arrangement.

|

|
|
Methane in two-dimensions
|
Methane showing the tetrahedral
3-D arrangement
|
Electronic shape and molecular shape
The shape of a molecule is given by the relative positions of its constituent atoms. This may, or may not, be the same as the electronic shape of the molecule. If all of the electrons around the central atom are involved in bonding, then the molecular shape equals the electronic orientation.
However, if there is one, or more, lone pairs (non-bonding pairs) of electrons, then the molecular shape will be different from the electronic orientation.
If, for example, the central atom has three electron domains (three charge centres), but is attached to only two atoms then the electronic shape is trigonal planar, but the molecular shape is angular (bent).
 |
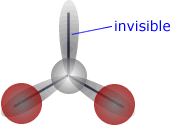 |
| Trigonal planar electronic shape - three charge centres | Angular molecular shape - 2 out of 3 charge centres used for bonding. |
The shape adopted by electron pairs around a central atom depends on the number of electron domains. Each domain will move so as to minimise the repulsive forces experienced. The molecular shape is not necessarily the same as the electronic shape, as only the positions of the atoms themselves are used to describe the molecular shape.
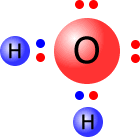
To determine the molecular shape it is necessary to first determine the shape adopted by the electrons, and only then can the positions of the atoms be known. The electronic shape is tetrahedral, but the shape of the water molecule considers only the H-O-H. It is angular, or bent. |
One of the successes of the Valence Shell Electron Pair Repulsion theory lies in its ability to predict, or explain, the bond angles of molecules. To do this, it considers that electron pairs that are shared by two atoms (bonding pairs) experience less repulsion than lone, or non-bonding pairs, of electrons. The logic of this assumption comes from the idea that shared pairs have their electron density relatively displaced away from the central atom by the bonded atom, when compared to a lone (non-bonding) pair.
This idea gives rise to an order or repulsive force experienced by electron pairs, in which lone pair - lone pair repulsion is greater than lone pair - bonding pair repulsion, which in turn is greater than bonding pair - bonding pair repulsion.
These different forces of repulsion distort the perfectly symmetrical shapes that would be adopted by the electron pairs under ideal conditions. We can appreciate this by studying examples such as the ammonia molecule, NH3.
Ammonia
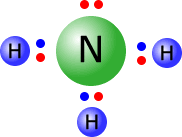
The ammonia molecule has a central nitrogen atom with four electron domains, each with a pair of electrons. These electrons tend to adopt a tetrahedral orientation. A perfect tetrahedron has a bond angle of 109º 28'.
However, there is a greater repulsion between the lone pair (non-bonding pair) of electrons and the bonding pairs, than there is between the bonding pairs themselves. This greater repulsive force has the effect of 'squeezing' the hydrogen atoms closer together, creating smaller H-N-H bond angles of 107º.
Water
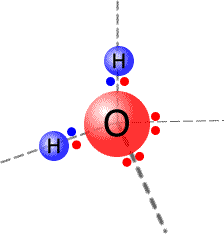
 The
water molecule has four pairs of electrons on the central oxygen atom. These
electron pairs, or electron domains, adopt a tetrahedral arrangement. The theoretical
bond angle of the electron pairs would be 109º 28'.
The
water molecule has four pairs of electrons on the central oxygen atom. These
electron pairs, or electron domains, adopt a tetrahedral arrangement. The theoretical
bond angle of the electron pairs would be 109º 28'.
However, only two of the electron pairs are actually used in bonding and the other electrons are lone (non-bonding) pairs. The lone pairs of electrons repel the bonding pairs more than the bonding pairs repel each other.
This has the effect of closing the 109º 28' bond angle down to 104º 30'.
Ions are atoms or groups of atoms that have lost or gained electrons to attain a stable electronic configuration. In the case of ions involving more than one atom the atoms always have an octet of electrons.
When counting up the total number of electrons available, it is important to remember that a positive ion has lost an electron and a negative ion has gained one or more electrons with respect to the constituent atoms.
For example, the ammonium ion has the formula NH4+. It is comprised of one N atom (group 15) and four hydrogen atoms. The total valence electrons from the atoms = 5 + 4 = 9. However, the ion has a positive charge, so it has lost a valence electron. It therefore has only 8 valence electrons.
The central nitrogen atom is attached to four hydrogen atoms by for shared pairs of electrons = 8 electrons. Thus there are no electrons left over.
Negative ions have electrons added, for example the NH2- ion. In this case the central nitrogen atoms provide 5 electrons and each hydrogen provides 1 electron. This makes a total of 7 valence electrons. However, the ion carries a negative charge that must be added into the valence shell. So the overall total of valence electrons is 7 + 1 = 8.
In all cases, ions have full octets in the valence shells.

