|
Compounds with benzene rings are known as aromatic. In reality there are other compounds as well as benzene derivatives that are aromatic, however at this level benzene serves as a good example of many of the bonding concepts covered. |
|
Benzene provides a good case study which examplified all of the preceding theories of bonding.
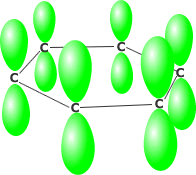
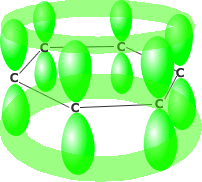
Benzene consists of six carbon atoms in a hexagonal ring, all of which are sp2 hybridised. This allows the perpendicularly oriented 'p' orbital of each carbon to overlap laterally all around the ring. The delocalised pi system produced leads to much greater stability than would be obtained by alternate double and single bonds.
 |
 |
|
Benzene's perpendicular 'p' orbitals
|
'p' orbital overlap resulting
in delocalisation
|
For many years there was doubt about the actual structure of benzene. It was possible to draw the structure with alternate double and single bonds, now known as a Kekule structure. Resonance ideas were evoked to suggest that there were two resonance Kekule forms that interchanged rapidly.
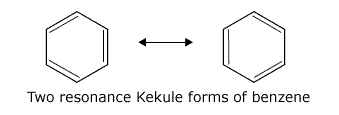
However, gradually evidence emerged that the actual structure of benzene involved delocalisation of electrons throughout the ring. Nowadays, the Kekule forms are used for mechanisms or simplicity when it is convenient to 'tie down' the electrons.
Evidence for the ring structure of benzene
1. Energetics
Comparison of the addition reaction of cyclohexene and benzene with hydrogen. If benzene were simply three alternate double and singel bonds (tri-ene) then hydrogenation of these three double bonds should release three times as much energy as hydrogenation of the one double bond in cyclohexene. In fact, hydrogenation of benzene releases far less energy, suggesting that benzene's structure is much more stable than that of a tri-ene.
2. Bond characteristics
Other evidence for the delocalisation of electrons in benzene emerges from consideration of the bond lengths. If benzene were to consist of alternate double and single bonds, then these would be different lengths. However, studies show that all of the benzene C-C bonds are the same length, intermediate between the lengths of C-C single and double bonds.
- C-C single bond length 0.154 nm
- C-C double bond length 0.134 nm
- C-C benzene bond length 0.139 nm
This is evidence that all of the carbon-carbon bonds in benzene are identical in terms of electron density.
The bond order could be described as 1.5, i.e. one single + one half bond.
3. Isomerism
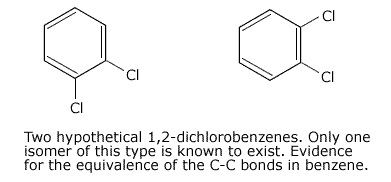 Absence
of the existence of 1,2 disubstituted isomers in benzene compounds provides
more evidence for a delocalised ring system.
Absence
of the existence of 1,2 disubstituted isomers in benzene compounds provides
more evidence for a delocalised ring system.
For example, if benzene were to have an alternating double and single bond structure, 1,2 dichlorobenzene could exist with a double bond, or a single bond between the carbon atoms holding the chlorine atoms.
The fact that only one form of 1,2-dichlorobenzene can be prepared indicates that all of the C-C bonds are equivalent.

