| Once it became clear that all matter is particulate in nature and that the fundamental particle is the atom (of which there are about 90 types that occur naturally), it was necessary to describe the constant composition of compounds in terms of the ratios of atoms that they contain. This leads to the idea that each atom is able to combine with a certain number of other atoms. This is called its valency. | 
|
The valency of an atom is the number of single chemical bonds that it can make (in the case of a covalently bonding substance) or the number of electrical charges that it carries (for an ion). Notice that once again the nature of the substance in question requires that the definitions be adapted appropriately. The concept of valence can be used to find the formula of a compound from the valencies of its constituent elements, or to find the valency of an elements within a compound of known formula.
Every atom within a substance is assigned a valency number that is either positive or negative. The total sum of all of the valencies within a formula unit is zero.
As can be seen from the table, it is much more difficult to predict the valencies of non-metals and transition metals, so it is always better to start with oxygen, hydrogen and group I/II metals when calculating valencies.
|
Example: Find the valency of the chlorine atoms in the compound Cl2O7 The oxygen has a valency of -2 7 oxygen atoms make a total of -2 x 7 = -14 The overall valency must cancel out, i.e sum of the valencies of oxygen + sum of the valencies of chlorine = 0 Therefore Cl2 = +14 Valency of chlorine in Cl2O7 = +14/2 = +7 |
To find whether an atom takes a positive or negative valency consider the electronegativity of the atom to which it is attached.
- In nitrogen dioxide, NO2, oxygen is MORE electronegative than the nitrogen and so the nitrogen takes a positive valency (in this case = +4).
- In ammonia, NH3, nitrogen is MORE electronegative than the hydrogen, so it is the nitrogen that takes the negative valency (in ths case -3).
|
Example: Find the valency of the nitrogen atoms in the compound hydrazine, NH2NH2 Nitrogen is more electronegative than hydrogen so the hydrogen takes its positive valency of +1 There are four hydrogen atoms = (4 x 1) = +4 The nitrogen atoms must cancel out the sum of the hydrogen's valencies Therefore 2N +4 = 0 The valency of the nitrogen atoms = -2 |
A covalent bond is a shared pair of electrons. When non-metals bond to other non-metals they always do so by sharing electron pairs. The total number of bonds that an atom has is called its valency.
In the water molecule oxygen combines with two hydrogens and so has a valency of 2.
A simple diagram of a water molecule makes this plain.
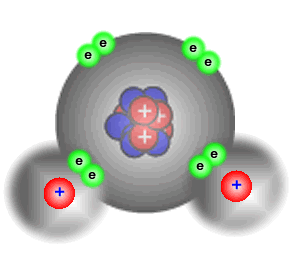 |
The hydrogen atoms are each sharing one pair of electrons - they have a valency of 1. The oxygen atom is sharing two pairs of electrons - it has a valency of 2. |
The rules of valency in compounds say that the total valency of the hydrogens must equal the valency of the oxygen. i.e. 1 + 1 = 2 If the most electronegative element is assigned a negative valency and the most electropositive element a positive valency then the sum of the atoms' valencies must equal zero. Hydrogen (electropositive) = +1 Oxygen (electronegative = -2 Sum: [2 x (+1)] + [1 x (-2)] = 0 |
|
Ionic substances are made up of giant ionic lattices. The simplest formula unit consists of the simplest ratio of oppositely charged ions. The total electrical charge MUST equal zero in a neutral compound.
|
The sodium chloride ionic structure consists of repeating sodium ions Na+ and chloride ions Cl-. The overall charge on the ionic structure is zero as the negative ionic charges are cancelled out by opposite positive charges. |
|
In this case the simplest ratio of ions is one sodium ion to one chloride ion and the formula unit is NaCl. Sodium has a valency of 1+ and the chloride ion has a valency of 1-. |
|
The valency of an ion is the number of electrical charges that it carries. A sodium ion has a single positive charge - it has a valency of +1. An oxide ion (from oxygen) has a charge of two minus, it has a valency of -2.
The same valency rules apply as for the covalent substances. The sum of positives must equal the sum of negatives.
|
Example: For the compound formed from sodium and oxygen. Sodium has a valency +1 Oxide (from oxygen) has a valency of -2 In order for the sum to equal zero we must have two sodium ions for each oxide ion. The formula = Na2O |
Using valencies
Once the valencies of a few elements are known it becomes a simple matter to construct the formula of unknown compounds using the valency method. Remember that the sum of the valencies of all of the atoms in the compound must equal zero.
Where an atom may have either positive or negative valency, it is negative if it is the more electronegative element in the compound and positive if not.
|
Example: From the water molecule above we know that the valency of hydrogen is +1. If the valency of nitrogen in ammonia is -3 then we can construct the formula of ammonia thus: We need enough hydrogens to cancel out the -3 valency of nitrogen. Each hydrogen = +1 therefore we need three hydrogen atoms. The formula of ammonia = NH3 |
Working with ions
When using valencies to work out the formula of an ion we have to remember the final charge on the ion must equal the sum of the valencies, taking into account whether the valency of each atom is negative or positive.
|
Example: Find the formula of the sulfate (2-) ion given that the valency of the sulfur atom is +VI and the valency of the oxygen atom is -II Oxygen always has negative valencies (unless bonded to fluorine) There is one sulfur atom with a valency of +6 and overall the ion has a valency of -2 Therefore +6 +(xO) = -2 Therefore (xO) = -2 -6 = -8 each O =-2 therfore there are four oxgen atoms in the ion Formula of the sulfate ion = SO42- |

