|
Titration is an experimental technique used to determine the concentration of an unknown solution. It usually involves acids and bases, but may be extended to any addition of one solution to another to find out the amounts required for reaction. |
|
General experimental procedure
The procedure involves adding one solution to another solution, in the presence of an indicator that shows when the two solutions have completely reacted. If the concentration and volume of one of the solutions is known and the volume of the other solution is known, then the unknown concentration can be calculated providing the stoichiometry of the reaction is known.
The experimental procedure is very accurate and liquids are carefully measured, using specially designed glass apparatus with low margins of error. See errors and inaccuracies.
Titrations are intended for analytical and determination/estimation purposes. If the compound being used for the titration cannot be obtained in a very pure state then it first must be standardised against a solution whose concentration can be determined to a high degree of accuracy. Such substances are called primary standards.
In summary:
- Measure 25cm3 of one solution into a conical flask
- Add a few drops of indicator solution
- Add a second solution very slowly from a burette until the indicator just changes colour
- Repeat the process until concordant results are obtained (results within 0.1 cm3 of one another)
|
Click on the 'titrate' button to see the general experimental procedure. Titration is carried out several times until results within 0.1cm3 of one another are obtained. These are then said to be 'concordant results' Readings on the burette should be taken at eye level; the bottom of the meniscus at the surface of the solution is usually read. Once concordant results have been obtained an average of these is used for calculations. |
|
|
Measure 25cm3 of one solution into a conical flask. Add a few drops of indicator solution. The reading on the burette is recorded taking into account the tolerance or error limits (e.g. ±0.05 cm3). |
|
Close to the 'end-point', i.e. the point at which the solute in the flask has been completely reacted, the solution is added from the burette 1 drop at a time, swirling the solution in the flask gently between each addition, until the colour just reaches the 'end-point'. |
 |
The solution is added slowly from the burette into the reagent solution in the flask until the colour of the indicator starts to change. |
 |
The final reading on the burette is recorded, once again taking into account the error limits. |
NOTE The solution in the conical flask does not have to be the unknown, the choice is usually dependent on the ease of observation of the indicator colour change. For example, it is easier to see the first hint of pink appearing from a colourless solution in a flask on a white tile, than to carry out the titration the other way round.
Calculations
The calculation is carried out as follows:
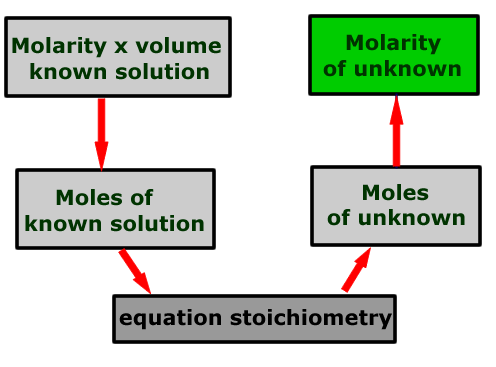
The number of moles of solute in the known solution is calculated using the relationship:
Then the stoichiometric relationship between this substance and the other reacting solution is used to determine the number of moles of solute in the unknown solution.
Example of equation stoichoimetry:
| Sodium hydroxide | + | nitric acid | |
sodium nitrate | + water |
| NaOH | + | HNO3 | |
NaNO3
|
+ H2O |
| 1 mole | ≡ | 1 mole |
Here the stoichiometric relationship is:
moles of base = moles of acid
The molarity of the unknown solution can now be calculated from the number of moles present and the volume used:
molarity = moles/volume(litres)
Calculation schematic
The calculation may be represented by a flow scheme showing the order of the operations to be carried out.
|
Example - Determination of the concentration of an unknown sodium hydroxide solution. Procedure
The potassium hydrogen phthalate reacts in a 1:1 ratio with sodium hydroxide Therefore at the end-point (equivalence point) Moles of potassium hydrogen phthalate = moles of sodium hydroxide molarity(acid) x volume(acid) = molarity(base) x volume(base) The molarity of the unknown sodium hydroxide solution may now be calculated. |