|
The relative reacting quantities in any chemical reaction are always the same, the ratio being determined by the stoichiometry of the equation for the reaction. On a microscopic scale we are dealing with particles reacting in whole number ratios. If there are more or less particles of one of the components reacting than needed, then this will not affect the ones actually reacting (undergoing chemical change). |
|
Excess reagent
When there is more of one of the reactants present than the required amount, the extra will not have anything to react with. This should be apparent bearing in mind the particulate nature of matter. If two molecules of hydrogen react with one molecule of oxygen to make two molecules of water, then any 'extra' molecules of hydrogen (or oxygen for that matter) will be unable to react.
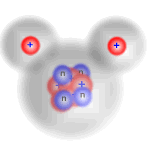
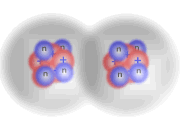
Three hydrogen molecules reacting with one oxygen molecule
|
|
 |
 
|
 |
||
|
Reactants
|
Products
|
Here you can see that the extra hydrogen molecule (at the lower right of the diagram) remains unreacted. We call this the excess reagent.
An extra amount of chemical over and above that which is needed for complete reaction is called the excess. A reagent in excess (i.e. one of which there is more than the amount needed) cannot completely react. Some of it can react, but the rest simply remains unreacted after the reaction has finished.
|
The principle: If there are 30 girls and 20 boys at a party then the maximum number of (heterosexual) couples that can be formed is 20 - it depends on the boys, who are in the minority. The equation would look like: 1 boy + 1 girl Hence: 20 boys 'react' with 30 girls The boys are the limiting reagent, they determine the number of couples that can be formed, and the girls are in excess. There are 10 girls that are going home disappointed. |
Calculating the excess
To find the excess reagent, the first stage is to calculate the number of moles of each reagent in the reaction. Then the stoichiometry of the equation shows the relative number of moles reacting in an ideal situation. The excess is found by substituting the number of moles of the first reagent (reacting chemical) in the given situation and seeing how many moles of the second reagent is required for complete reaction.
If there is more than enough of the second reagent then it is in excess. If there is not enough of the second reagent then the first reagent is in excess.
|
Example: 64 grams of sulfur react with 64 grams of iron Fe + S 1 mole of iron reacts completely with 1 mole of sulfur. RAM Fe = 56, RAM S=32. From the quantities given we have 64/32 moles of sulfur and 64/56 moles of iron i.e. 2 moles of sulfur and 1.143 moles of iron But as they always react in a 1 : 1 mole ratio then 2 moles of sulfur would need 2 moles of iron. Clearly there is not enough iron to react with all the sulfur. Therefore some of the sulfur must remain unreacted once all of the iron is used up. The sulfur is said to be in excess. Only 1.143 moles of the sulfur can react with 1.143 moles of iron. This means that 2 - 1.143 moles of sulfur is left unreacted = 0.857 moles |
Limiting reagent
In the above example it can be seen that the component that determines the amount of sulfur that can react is the iron. All of the iron manages to react and it is this that determines the quantity of products formed. The iron is said to LIMIT the reaction. It is called the limiting reagent.
To determine the limiting reagent (and to find out which of the reactants is in excess) the stoichiometry of the reaction must be considered.
Procedure
- Firstly find the relative number of moles of each component in the balanced equation.
- Then convert the data given in the question under study into moles.
- Now by inspection see which one of the components will completely react and which one will be in excess.
|
Example: 25cm3 of 0.2 M sodium hydroxide solution is mixed with 80 cm3 of 0.05 M sulfuric acid. 2NaOH + H2SO4 From the equation 2 moles of sodium hydroxide react completely with 1 mole of sulfuric acid From the data given: 25cm3 of 0.2 M sodium hydroxide = 0.025 x 0.2 moles = 0.005 moles 80 cm3 of 0.05 M sulfuric acid = 0.08 x 0.05 = 0.004 moles From the equation mole ratios, 0.005 moles of sodium hydroxide would react completely with 0.005/2 = 0.0025 moles of sulfuric acid. But there are 0.004 moles of sulfuric acid (i.e. more than enough). Some of the sulfuric acid will remain unreacted - it is in EXCESS. The LIMITING reagent is the sodium hydroxide as it is this alone that determines the amount of product formed |