| Neutralisation is the reaction between an acid and a bases. Neutralisation is nearly always an exothermic process. |
|
Definition
The energy released when 1 mole of water is formed by the reaction between an acid and a base in aqueous solution.
|
HCl(aq) + NaOH(aq)
|
Ions in aqueous solution and strong acids are completely dissociated, so the equation may also be written:
|
H+(aq) + OH-(aq)
|
Here it may be seen that the reaction does not depend on the nature of the acid or base (providing that they are both completely dissociated)
| The enthalpy change of neutralisation for strong acids and bases = -57 kJ |
Weak acids and bases
Weak acids are not completely dissociated in solution. Ethanoic acid is only about 1% dissociated according to the equation:
|
CH3COOH
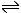 CH3COO- + H+
CH3COO- + H+ |
In order for the hydrogen ions to react with hydroxide ions, the ethanoic acid molecules must first dissociate; this requires energy. The overall energy change for the neutralisation reaction is the energy released by the formation of the water molecules minus the energy required to dissociate the molecules of the weak acid.
In reality, the differences in energy between weak and strong acids when neutralised are not large, but they are measureable. The weaker the acid, the lower the value of the enthalpy change.
|
Example: Calculate the enthalpy of neutralisation of ethanoic acid if 25cm3 of 1 M ethanoic acid and 25cm3 of 1M NaOH are mixed together and the temperature rise recorded is 6.2ºC (assume that the specific heat capacity of the mixture is 4.2 kJ kg-1 ºC-1 and the density of the mixture is 1g cm-3) Total mass of mixture = 25 + 25 = 50g = 0.05kg
E = 0.05 x 4.2 x 6.2 = 1.302
kJ
Number of moles of water produced in the reaction: CH3COOH + NaOH
is equal to the number of moles of acid reacting = 1.0 x 0.025 = 0.025 moles Therefore energy per mole = 1.302/0.025 = 52.1 kJ mol-1 |
Experimental determination
The experimental determination of neutralisation enthlapy is one of the simplest experiments you are likely to come across. It is a straightforward matter of mixing a solution of the chosen acid with known volume and concentration with a suitable quantity of the chosen base, in an insulated container, such as a polystyrene beaker with a lid.
The reaction is almost instantaneous, so the heat generated goes to increasing the temperature of the mixture and little is lost to the surroundings.
Procedure:
- 1. Weigh a polystyrene beaker and lid.
- 2. Measure 50cm3 of 2 mol dm-3 hydrochloric acid into a polystyrene beaker. Record the temperature,and the mass.
- 3. Measure 50cm3 of 2 mol dm-3 sodium hydroxide into a polystyrene beaker. Record the temperature, and the mass.
- 4. As the two volumes are the same, the final temperature without reaction would be the average of the two temperatures.
- 5. Quickly mix the contents in one of the beakers and record the highest temperature reached.