|
The aim of scientists is to understand and manipulate the world around them. This section summarises how reaction rates are affected by changing conditions. |
|
Concentration of reactants
SL requirement
Increasing the concentration of a reactant increases the rate of reaction due to the greater number of collisions between reactant particles.
HL requirement
The concentration appears in the experimentally determined rate equation:
Rate = k [A]x[B]y
Remember that if the order with respect to a component is zero, then changing the concentration has no effect on the rate.
Particle size
SL requirement
For solids the smaller the particle size the faster the reaction. Powders are the solids with the smallest particle size.
Liquids, solutions and gases react much faster than solids as their particles are much smaller.
Decreasing the particle size increases the available surface area available for reaction.
Smaller pieces of solid have a greater surface area, more collisions at this surface and a faster rate.
HL requirement
If one of the reactants is in the form of a solid, then it will not appear in the rate equation, as the rate equation only shows concentrations and solids cannot have a concentration (being in a different phase).
However, the size of the solid lumps has an effect on the rate of reaction due to the available surface area for collisions.
Temperature
SL requirement
Increasing the temperature increases the energy of the particles (on average) and increases the likelihood of successful collisions.
This is because all reactions have an energy barrier, the activation energy. This is the minimum energy that colliding particles must have before they can react.
When the temperature is increased there are more particles with the required activation energy. A rough guide is that the rate of a reaction doubles for every 10º rise in temperature.
There are also more collisions although this is of less importance.
HL requirement
Temperature is related to the rate constant via the Arrhenius equation:
k = Ae-Ea/RT
Where Ea is the activation energy, R is the universal gas constant and T is the absolute temperature in Kelvin. A is the Arrhenius (frequency) factor.
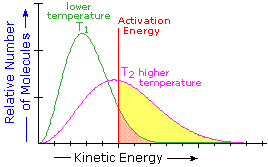 The equation is derived from the Maxwell - Boltzmann graph of energy distribution
in particles.
The equation is derived from the Maxwell - Boltzmann graph of energy distribution
in particles.
The term e-Ea/RT gives the number of particles under the curve that have energy greater then the activation energy, Ea.
These are the particles that can react if the orientation of a collision is correct.
Catalysts
SL requirement
Catalysts provide an alternative mechanism for reaction that has a lower activation energy and therefore a faster rate. Once a catalyst is used we are actually dealing with a different reaction, even though the reactants and products remain the same.
Catalysts may be heterogenous or homogeneous in which case their mode of action is somewhat different.
Heterogeneous catalysts provide a template surface at which reaction can occur, whereare homogeneous catalysts form intermediate products that release the catalyst during a subsequent step of the mechanism.
Other factors
There may be other factors that affect certain reactions such as those that are light sensitive.
A photographic film does not react in the dark, but does as soom as it is exposed to light.
Similarly plants cannot photosynthesise carbon dioxide and water to manufature carbohydrates without sunshine. In both cases the energy from the light stimulates the reaction process. It could be said that the reaction is light catalysed although this is not a true use of the term catalysis.
Light waves in these reactions causes electrons in the molecular orbitals to be promoted to higher orbitals weakening bonds. These weakened bonds then have a much lower activation energy for reaction. This can occur because the energy of the light is equal to the energy difference between the specific molecular orbitals.
|
Example: The reaction between hydrogen gas and chlorine gas is light catalysed. In a darkened room the gases can be mixed in a glass container. If the glass container is then exposed to sunlight the gases immediately explode. (don't try this!) H2 + Cl2 |

