|
Syllabus ref: 7.1 Syllabus ref: 17.1 The 'position' of equilibrium is a concept that describes the extent of a chemical equilibrium from the point of view of the amount of reactants, 100% initially, and products. |
|
Equilibrium position
This is defined as the state of the reaction mixture, with respect to the reactants and products. The equilibrium law gives us a measure of the position of equilibrium.
The equilibrium law expression: 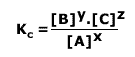
For the general reaction: xA ![]() yB + zC
yB + zC
Shows us that as the product concentration increases so does the value of Kc. Hence, for a reaction that lies well to the left hand side at equilibrium, the value of Kc is very small. Conversely, for a reaction that proceeds almost to completion and lies well to the right hand side, the value of Kc is very large.
We can assess the position of equilibrium by considering the value of Kc.
| Value of Kc | Position of equilibrium |
| >>1 | far to the right hand side |
| <<1 | far to the left hand side |
| 1 | approximately in the middle |
Equilibrium and Gibbs free energy
The question now arises, "why do some reactions proceed at first and then stop at the equilibrium position?"
We know that the driving force behind any process is the increase in universal entropy and it follows that this must be the case for equilibrium systems. The equilibrium position is the state in which the universal entropy is at a maximum and hence Gibbs free energy is at a minimum.
Gibbs free energy change is given by the formula:
ΔG = ΔH - TΔS
and at equilibrium the system no longer changes, therefore ΔG = 0.
The Gibbs free energy change of a reaction and the equilibrium constant can both be used to measure the position of an equilibrium reaction and are related by the equation:
ΔG = -RTlnK

