|
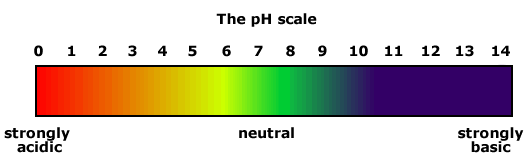
The pH scale provides us with a method of describing the degree of acidity, or basicity of a solution. |
|
The pH scale
The term 'pH' derives from the french for 'potential hydrogen'. The pH scale ranges from 0 to 14.
The pH is a measure of the concentration of hydrogen. It is defined by the negative of the logarithm of hydrogen ion concentration:
![]()
The higher the hydrogen ion concentration the lower the pH value. pH can be measured with a pH meter, or with pH paper (paper containing a mixture of indicators to cause a continuous color change).
| pH | [H+] / mol dm-3 |
|---|---|
| 1 | 0.1 |
|
2
|
0.01
|
|
3
|
1 x 10-3
|
|
4
|
1 x 10-4
|
|
5
|
1 x 10-5
|
|
6
|
1 x 10-6
|
|
7
|
1 x 10-7
|
|
8
|
1 x 10-8
|
|
9
|
1 x 10-9
|
|
10
|
1 x 10-10
|
|
14
|
1 x 10-14
|
As can be seen in the table, a change in pH value of 1 unit is equivalent to a ten-fold change in hydrogen ion concentration.
|
Example: How many times greater is the hydrogen ion concentration of a solution with pH=1 than a solution with pH=3? pH1 has a hydrogen ion concentration of 1 x 10-1 mol dm-3. (0.1 mol dm-3) pH3 has a hydrogen ion concentration of 1 x 10-3 mol dm-3. (0.001 mol dm-3) Therefore pH1 has a hydrogen ion concentration 100 times greater than pH3. |
Strongly acidic
pH 1 represents strong acid, getting progressively weaker as the pH rises. pH 7 is the neutral value of pure water at 25ºC and 1 atmosphere pressure.
If we have two solutions with their pH values, the lower one will be more acidic and the higher one will be more basic (though they could both still be basic/acidic with respect to water at pH 7).
Highly basic
Solutions with a pH of greater than 13 are highly basic. They show blue/purple to universal indicator and blue to litmus.
Highly basic solutions include sodium hydroxide(aq), potassium hydroxide(aq) and barium hydroxide(aq). Such solutions are often said to be 'caustic'. For example 'caustic soda' is an old term for sodium hydroxide. A 0.1 mol dm-3 solution of sodium hydroxide has a pH of 13.
Neutral
Generally considered to be pH 7. This is the pH of pure water at 25ºC. Neutral solutions have no effect on litmus paper and turn Universal indicator green.