| Electroplating, or electrodeposition, is the process of applying a layer of metal to the surface of another metal. It is a variation on the theme of electrolysis. There are many applications of this process in use in the modern world. | 
|
Participating electrodes
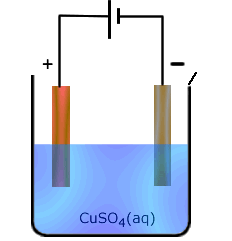
When non-inert electrodes are used in electrolysis they can interact with the solutions being electrolysed. This specifically is important when electrolysis of ions of relatively unreactive metals are electrolysed using the same metal as an electrode. In this case the anode can participate in the reaction by losing atoms in the form of ions to the solution, leaving behind their own electrons.
This is probably best understood by looking at an example. In the electrolysis of copper(II) sulfate solution using copper electrodes. The reaction that would proceed at the anode with inert electrodes would be:
| 4OH-(aq) - 4e |
This requires migration of hydroxide ions, which are at a fairly low concentration, to the anode for liberation. The alternative possibility in the case of a copper anode, is for the following reaction to take place:
| Cu(s) - 2e |
This second reaction has the advantage of not requiring any migration (the copper atoms are already there, they make up the electrode), and the reaction itself is energetically more advantageous. For both of these reaons, the second reaction takes preference.
Consequently, the following reactions occur:
At the anode (positive electrode): Cu(s) - 2e ![]() Cu2+(aq)
Cu2+(aq)
At the cathode: (negative electrode) Cu2+(aq) + 2e ![]() Cu(s)
Cu(s)
The overall effect is for copper ions to be liberated as copper at one electrode at the same time as they are being produced by the other electrode. The concentration of copper(II) in solution does not change. However, the mass of copper at the anode decreases and the mass of copper at the cathode increases.
This is summarised in the following table:
| Electrolysis of a copper(II) solution using copper electrodes | ||
|---|---|---|
|
solution
|
anode
|
cathode
|
|
[Cu2+(aq)] unchanged
|
mass Cu decreases
|
mass Cu increases
|
|
Cu
|
Cu2+ + 2e
|
|
The purification of copper
The participating electrode effect can be put to good use in the purification of copper. Impure copper can be used as the anode in an electrolysis cell and pure copper gets deposited at the cathode. The concentration of copper ions in the electrolyte remains unchanged.
 |
The reaction at the anode:
Reaction at the cathode
|
Electroplating
Any metal lower than hydrogen in the electrochemical series, i.e. with a positive electrode potential, can be deposited at the cathode during electrolysis. This means that these metals can be used to electroplate other substances. The requirements for successful electroplating are quite stringent in that the deposit of metal often fails to adhere successfully to the material of the cathode, unless the conditions are optimised.
Suitable electroplating metals include:
- Copper
- Chromium
- Nickel
- Silver
Chrome plating
Chromium is used in many areas as a decorative coating due to its bright silver colour and shine. There are two electrolytic processes used for chrome plating, which differ only in the electrolyte used. There are advantages and disadvantages in both processes.
In the hexavalent chromium process, the electrolyte used is chromium(VI) oxide dissolved in sulfuric acid. This produces a highly acidic and toxic mixture. In the trivalent chromium process the electrolyte is chromium(III) sulfate or chromium(III) chloride.
Both processes involve three phases:
- 1 Activation
- 2 Chromium electroplating
- 3 Rinsing
The activation stage involves etching and cleaning the surface of the metal to be electroplated, so that the layer of chromium adheres tightly to the surface. It typically takes place in a bath of chromic acid, using reverse current to remove surface impurities.
 The electrolytic
stage the current and temperature are carefully controlled to give the coating
characteristics required. In the chromium(III) process additives must be used
to prevent oxidation taking place at the anode.
The electrolytic
stage the current and temperature are carefully controlled to give the coating
characteristics required. In the chromium(III) process additives must be used
to prevent oxidation taking place at the anode.
The main disadvantages of the hexavalent process is its cost and toxicity (The film Erin Brockovich is a true story about a woman pursuing compensation from a company that polluted the land with hexavalent chromium causing health problems and morbidity in the local population). The final product is not toxic, as the rinsing procedure leaves only pure chromium.
The disadvantage of the trivalent process is that the colour of the chrome plate is not always up to customer expectations. However, this problem has been resolved by the use of certain additives. The plating is generally more even than the hexavalent process.

