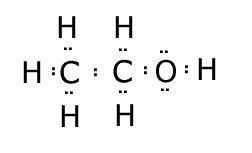|
Representation of chemical formula in organic chemistry must leave the structure unambiguous. There are several accepted ways to do this. |
Empirical formula
The empirical formula is the simplest ratio of the atoms within a molecule of the compound. It emerges from calculations of formula using a consideration of the percentage composition by mass of each element.
Traditionally, the method of determining the nature of a substance was based on a direct analysis of the elements within the compound, taking advantage of the fact that most organic compounds are flammable. When burnt, all of the carbon turns to carbon dioxide and all of the hydrogen turns to water. Thus, if the mass of carbon dioxide produced from a known mass of an unknown compound is found, the actual mass of carbon and hence the percentage carbon in the original compound can be calculated.
|
Example: 5g of an unknown organic compound produced 11.0g of carbon dioxide on complete combustion in excess air. Calculate the percentage carbon in the compound. 11.0g of carbon dioxide, formula CO2, relative mass = 44 ∴ 11.0g contains 12/44 x 11.0g of carbon = 3.0g Percentage carbon in the unknown compound = 3/5 x 100 = 40.0% carbon |
The same process can be applied to the hydrogen in an organic compound as it all turns to water.
|
Example: 5g of an unknown organic compound produced 3.3g of water on complete combustion in excess air. Calculate the percentage carbon in the compound. 3.3g of water, formula H2O, relative mass = 18 ∴ 3.3g contains 2/18 x 3.3g of carbon = 3.35g Percentage carbon in the unknown compound = 0.37/5 x 100 = 6.7% hydrogen |
Molecular formula
The molecular formula shows the actual number of atoms of each type in the molecule. For example, the molecular formula of ethanol is C2H6O.
There are two fundamental problems with using the molecular formulae to represent molecules:
- They give no indication as to the actual arrangement of the atoms within the molecule.
- There may be many different molecules with the same molecular formula.
However, in analysis the molecular formula is a good start when working out the identity of a compound. A high definition Mass Spectrometer can determine the relative formula mass of a compound to such a high degree of accuracy that the molecular formula can be obtained directly. It is then up to other techniques to identify the actual arrangement of the atoms within the molecule.
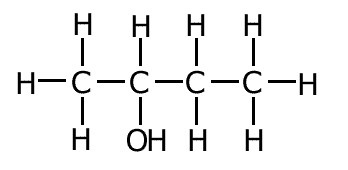
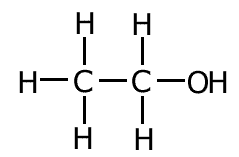
Structural formula
The structural formula shows the actual arrangement of the atoms in a molecule by drawing the bonds as lines between letters representing the atoms. A single bond is show as one line only and a double bond is shown as a double line.
In this case there is rarely any problems of ambiguity when representing molecules. Consequently the structural formula is the representation of choice in most cases.
|
Example: The structural formula of ethanol C2H6O is represented as follows:
|
Condensed formula
The condensed formula is a shorthand method of representing the structural formula which relies on some knowledge of chemical structures. The structure is written starting at one end of the chain with each carbon shown along with any attachments. There are no single bonds shown between carbon atoms, as it is assumed that the reader understands that the atoms must be joined together in the chain, using at least one bond.
Ethanol is represented as CH3CH2OH. Reading from left to right this says:
- Carbon number one is attached to three hydrogen atoms.... then joined to carbon number two, which
- has two hydrogen atoms attached and is joined in turn to...
- an oxygen atom which also has one hydrogen attached.
Where groups are attached to the carbons in the chain they can be show by using brackets immediately after the carbon to which they are attached.
|
Example: CH3CH(OH)CH2CH3 In this case there is an -OH group attached to carbon number 2 in the chain.
|
Don't forget that carbon always forms four bonds. This can help when drawing a structure. If you end up with a carbons with 3 or 5 bonds then you have done it wrong!
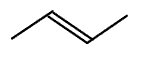
Skeletal formula
This is another way to represent the molecular structure. In a skeletal formula each carbon is represented by an angle, or termination in a line and the hydrogen atoms are just assumed. Double bonds are shown as a double line and hetero atoms are draw as usual. A certain amount of logical reasoning must be used to understand the structure from a skeletal representation.
|
butane
|
trans-but-2-ene
|
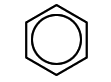
benzene
|
 |
 |
 |
.gif) |
.gif) |
.gif) |
This type of representation is very useful when the molecule is large and the number of atoms becomes too unwieldy for other representations.
NOTE The IBO will NOT accept skeletal formula to represent structures. If you are asked for a structure you MUST show all of the atoms.
Lewis structures
These are structural formula in which the bonds are not represented by lines, but rather by the electron pairs that make up the bonds. Any lone (non-bonding) electron pairs must also be shown. The electron pairs may be shown as two dots, or two crosses, or even a line.
|
Example: The Lewis structure of ethanol C2H6O is represented as follows:
Notice that the two lone pairs on the oxygen are also shown. When drawing Lewis structures there are nearly always eight electrons around each atom (except hydrogen). There are some exceptions, but not in organic chemistry. |