|
Alcohols are an homologous series of organic compounds containing the hydroxyl group. The commonly known 'alcohol' is actually more correctly known as ethanol. Industrially, in recent years ethanol was manufactured in the petrochemicals industry, however nowadays it is also produced as a biofuel by fermentation. |
|
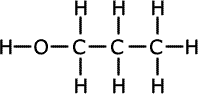
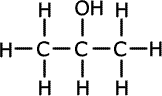
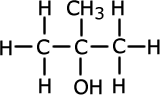
Alcohols (also called alkanols) are organic compounds that contain an OH group of atoms directly attached to the carbon chain. Alcohols have an -OH group of atoms attached to the carbon chain. They may be primary, secondary or tertiary depending on the number of carbons bonded to the carbon atom that holds the -OH group.
 |
 |
 |
|
Propan-1-ol (primary) |
Propan-2-ol (secondary) |
Methylpropan-2-ol (tertiary) |
Alcohols, like the majority of organic compounds, burn in air or oxygen. The products of complete combustion are carbon dioxide and water. In a restricted supply of air the products will include carbon monoxide and possibly carbon as well as carbon dioxide and water.
Complete combustion:
|
methane + oxygen CH4 + 2O2 |
Alcohols are oxidised by strong oxidising agents such as potassium dichromate (VI) in acidic solution. The products depend upon the nature of the alcohol.
- Primary alcohols produce aldehydes
- Secondary alcohols produce ketones
- Tertiary alcohols are not easily oxidised (no reaction with potassium dichromate in acidic solution)
This reaction may be used to differentiate between 1º, 2º and 3º alcohols.
Oxidation of primary alcohols first produces aldehydes, which may be further oxidised to carboxylic acids under the same conditions. This means that to obtain the aldehyde from this reaction it must be removed as soon as it is formed. This is done by making use of the fact that aldehydes are more volatile than the corresponding alcohol and carboxylic acid.
|
|
Oxidation of secondary alcohols produces ketones (alkanones). Ketones can't be further oxidised, so this reaction is carried out in reflux apparatus.
|
|
Summary - The oxidation of alcohols
Alcohols react with carboxylic acids producing esters. These are compounds containg an ester linkage -COO- between two alkyl chain segments.
|
methanol + ethanoic acid CH3OH + CH3COOH |
The reaction is catalysed by concentrated sulfuric acid, which also absorbs the water formed, pulling the equilibrium to the right hand side. The name of the ester formed is derived from the alkyl chain of the alcohol first followed by the acid derived part.
- Methanol makes methyl esters
- Ethanoic acid makes ethanoates
Esters are fruity smelling liquids that are used in the food industry as flavorings and scents.
Dehydration reactions (IB options only)
Alcohols with two or more carbons can be dehydrated (the elements of water, H2O, removed) by heating at 170ºC with concentrated phosphoric acid, sulfuric acid or by passing over hot aluminium oxide.
Ethanol loses H2O and produces ethene:
|
ethanol CH3CH2OH |
Butan-2-ol can produce two different isomers:
|
CH3CH(OH)CH2CH3 CH3CH(OH)CH2CH3 |
The mechanism proceeds via protonation of the oxygen atom, followed by loss of a water molecule and electron rearrangement, releasing a proton.
If the temperature is not too high, 140ºC, then a different dehydration reaction predominates, producing ethers.
|
2CH3CH2OH |


