| The characteristics, or properties of bonds formed by sharing electron pairs is examined in this section. | 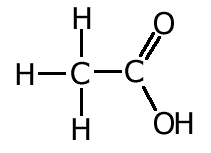
|
Single bonds and the octet rule
As stated in the previous section, single bonds consist of a shared pair of electrons. Each bonded atom usually provides one electron for the shared pair. The result is a full outer shell of electrons. This is known as the octet rule. This rule suggests that covalent bonds form so as to provide each atom in the molecule with a full outer shell of electrons, usually eight, therefore 'octet' (group of eight).
 |
Each fluorine atom provides one electron to the shared pair. The fluorine atoms both now have a full outer shell (octet) of electrons |
|
Fluorine molecule
|
Usually, the two atoms involved in a covalent bond provide one electron each to make the pair. However, occasionally, both of the electrons come from one of the atoms. In this case, the bond is said to be dative (= giving) covalent. The fact that an electron pair is dative has no influence on the final structure.
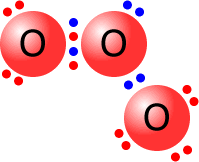 |
The central oxygen atom is bonded to one of the other oxygen atoms by a dative covalent bond (blue pair). All of the oxygen atoms have a full outer shell (octet) of electrons. |
|
Ozone molecule
|
Double bonds consist of two shared pairs of electrons between the bonded atoms.
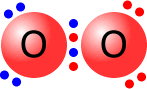 |
The two single bonds are actually different in character and are given names to differentiate them, sigma and pi bonds. A double bond always consists of 1 sigma bond and 1 pi bond. |
|
Oxygen molecule
|
Triple bonds consist of three shared pairs of electrons between the bonded atoms. These bonds are not all the same and consist of one 'sigma' bond and two 'pi' bonds.
 |
In this case one of the 'pi' bonds is formed by donation of one pair of electrons from the oxygen to the bond. It is a dative bond. |
|
Carbon monoxide molecule
|
There are exceptions to the octet rule, such as NO and NO2, in which the nitrogen atom does not have a full outer shell. The molecule is said to be electron deficient, as it is missing an electron.
 |
Nitrogen has only seven electrons in its outer shell. It is electron deficient. Molecules such as this are usually very reactive. |
|
nitrogen(II) oxide molecule
|
Electron deficient molecules can be identified by counting up all of the available valence electrons. If they add up to an odd number then there MUST be one electron left over somewhere, as an unshared single electron.
In the case of the nitrogen(II) oxide molecule (diagram above), the formula is NO. Nitrogen is from group 15 and has five valence electrons. Oxygen is from group 16 and has six valence electrons.
Total valence electrons = 5 + 6 = 11
This is an odd number and so there has to be an unpaired electron somewhere.
The strength of the bond depends very much on the atoms being bonded. However, carbon forms single, double and triple bonds with other carbon atoms and we can use this to determine the relative strength of these bonds.
|
Bond type
|
Bond strength
|
| C - C | 348 kJ mol-1 |
| C = C | 612 kJ mol-1 |
| C |
837 kJ mol-1 |
Single bonds use one pair of electrons to hold atoms together. Greater force of attraction between the electron pairs and the two nuclei draw the atoms closer together reducing the bond length. The values in the table below are given in nm (nanometres) = 1 x 10-9 m
|
Bond type
|
Bond length
|
| C - C | 0.154 nm |
| C = C | 0.134 nm |
| C |
0.120 nm |
This is not just the case for carbon carbon bonds, the principle applies to all single and multiple bond systems; i.e. a carbon - oxygen single bond is longer than a carbon - oxygen double bond.
|
Bond type
|
Bond length
|
| C - O | 0.133 nm |
| C = O | 0.122 nm |
| C |
0.111 nm |
We can make use of these facts to make logical deductions about the nature of bonding in certain molecules.
For example, studies show that the carbon - oxygen bond lengths in carboxylic acids is different for the two bonds. This can be explained by the fact that one of the bonds is a double bond whereas the other is a single bond.
However, bond lengths measured in the carboxylate ion, indicate that both C-O bonds are the same length. The reason for this is dealt with in the resonance section.

