|
Lewis structures are diagrams of molecules that represent electron pairs by dots, crosses or lines. They show all of the valence electrons, including lone, or non-bonding pairs. |
|
Otherwise known as Lewis structures, dot-cross representations show the electrons in the outer shell (valence electrons) of all of the atoms involved in a molecule. Covalent bonds consist of electron pairs and there are also lone, or non-bonding pairs.
Conventionally, either dots or crosses can be used to represent the electrons in each outer shell:
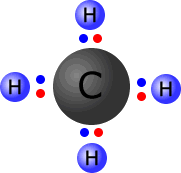
In the image at the left, the four hydrogen atoms are bonded to the central carbon atom by means of covalent bonds. Each bond consists of a pair of electrons, conveniently coloured to distinguish those electrons that 'originally' came from carbon from those that 'belong' to the hydrogen atoms.
Atoms (with the exception of the noble gases) are not stable on their own. They MUST bond to produces structures in which the electronic arrangement is stable. In 99% of cases this means that the outer shell has a full 'octet', or eight electrons.
The number of outer shell electrons may be found from the position of the
atom in the periodic table. For instance, fluorine is in group 17 and so it
has seven outer (valence) electrons.  All
single covalent bonds are represented by a pair of electrons, each bonding
atom providing one for the pair. There are two fluorine atoms in the fluorine
molecule and so there are 15 electrons altogether. Two electrons are used
in the single F-F bond leaving 12 electrons to share over the two atoms. These
are arranged as three non-bonding pairs per atom.
All
single covalent bonds are represented by a pair of electrons, each bonding
atom providing one for the pair. There are two fluorine atoms in the fluorine
molecule and so there are 15 electrons altogether. Two electrons are used
in the single F-F bond leaving 12 electrons to share over the two atoms. These
are arranged as three non-bonding pairs per atom.
|
Example: Draw the Lewis structure of the ammonia molecule. Ammonia has the formula NH3, therefore there is one N atom and three H atoms.
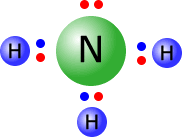
Total outer electrons = 3 + 5 = 8 Each N-H bond requires 2 electrons, therefore 3 x N-H = 6 electrons used in the three bonding pairs. Remaining electrons = 8 - 6 = 2 electrons, therefore one non-bonding pair. |
A double bond is shown as two shared pairs of electrons, each of the bonded
atoms provide two electrons for the bond. In the oxygen molecule at the left,
their are two shared pairs of electrons giving a stable octet (eight) of electrons
around each oxygen atom. 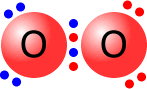
In triple bonds there are three pairs of electrons holding the two atoms
together. The molecule shown at the right is the ethyne (acetylene) molecule.
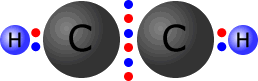 Each
of the carbon atoms (group IV) originally started with 4 outer electrons.
In the ethyne molecule at the right, both of the carbon atoms now have a full
octet (eight) of electrons and the two hydrogen atoms have a full first shell,
with two electrons. There are no non-bonding pairs.
Each
of the carbon atoms (group IV) originally started with 4 outer electrons.
In the ethyne molecule at the right, both of the carbon atoms now have a full
octet (eight) of electrons and the two hydrogen atoms have a full first shell,
with two electrons. There are no non-bonding pairs.
These are strucures in which the outer (valence) shells do not have a full
octet of electrons. This may be because the molecule has been formed from
electropositive atoms that have few electrons in the outer shell, or possibly
the molecule is just an exception. Such molecules are by definition unstable
and there are few exceptions that need to concern us here.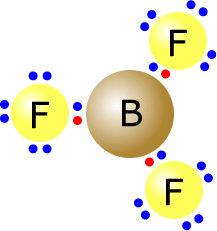
Boron trifluoride is electron deficient, as the boron atom is relatively electropositive and only has three outer electrons. The fluorine atoms themselves do have a full outer shell, but the boron atom in the centre has only six electrons in its valence shell.
Another example of electron deficiency is the beryllium dichloride molecule, BeCl2.
To construct the Lewis structure of beryllium dichloride we have to first
consider the number of outer electrons in the central atom Beryllium is from
group II and has two electrons in the outer shell. It bonds to two chlorine
atoms. 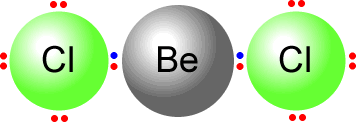 Chlorine
can only use a single bond when bonding as it has 7 electrons in its outer
shell. There are two chlorine atoms therefore the beryllium shares two pairs
of electrons, one with each chlorine. However this then leaves beryllium with
only four electrons in its outer (valence) shell. It is electron deficient.
Chlorine
can only use a single bond when bonding as it has 7 electrons in its outer
shell. There are two chlorine atoms therefore the beryllium shares two pairs
of electrons, one with each chlorine. However this then leaves beryllium with
only four electrons in its outer (valence) shell. It is electron deficient.
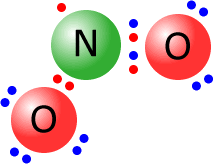 Occasionally
molecules may manage to become stable even though they do not have a full
outer shell. This is not common, but is the case for nitrogen(IV) oxide, NO2,
at elevated temperatures. As you can see from the Lewis structure, there are
only seven electrons around the central nitrogen.
Occasionally
molecules may manage to become stable even though they do not have a full
outer shell. This is not common, but is the case for nitrogen(IV) oxide, NO2,
at elevated temperatures. As you can see from the Lewis structure, there are
only seven electrons around the central nitrogen.
It is impossible to draw a Lewis structure in which all of the atoms are surrounded by an octet of electrons. However, the molecule does exist. It is reactive and at lowish temperatures soon pairs up to form a more stable structure, N2O4, in which all of the atoms do have a full octet.
| 2NO2 |
Ions are atoms or groups of atoms that carry a formal electrical charge. This charge may be positive or negative. Negative charges mean that the atoms, or groups of atoms have gained extra electrons to form a stable structure. Positive ions, conversely, have lost electrons in order to attain a stable electronic structure.
 For
example, a chlorine atom has the electronic configuration 2, 8, 7. It does
not have a full outer shell and when on its own is extremely reactive - it
is called a free radical. It's extreme reactivity is due to the instability
of an incomplete outer shell.
For
example, a chlorine atom has the electronic configuration 2, 8, 7. It does
not have a full outer shell and when on its own is extremely reactive - it
is called a free radical. It's extreme reactivity is due to the instability
of an incomplete outer shell.
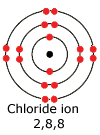 A
chloride ion has one extra electron needed to complete the outer shell. Consequently
it carries a negative charge.
A
chloride ion has one extra electron needed to complete the outer shell. Consequently
it carries a negative charge.
All simple ions can be thought of as the result of the atom needing to gain or lose electrons to make up a full outer shell octet. Ions that consist of two or more atoms can be treated in exactly the same way.
 A
multi-centre ion consists of two or more atoms that have gained or lost electrons
leaving a stable electronic structure.
A
multi-centre ion consists of two or more atoms that have gained or lost electrons
leaving a stable electronic structure.
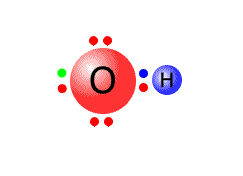
For example, the hydroxide ion has the formula OH-. It is made up of one oxygen atom and one hydrogen atom, which together have 6 + 1 = 7 valence electrons. There is a negative charge on the ion, meaning that the two atoms have gained one more electron, making a total of 8 electrons. The O-H bond requires one pair of electrons, leaving 6 to be distributed over the oxygen and hydrogen atoms.
The Hydrogen atom does not need any more electrons as it already has a share of 2 in the outer shell. The remaining 6 electrons therefore can go onto the oxygen atom as three non-bonding pairs.
In the diagram, the extra electron is show in green, although in reality there is no way of distinguishing it from the others.
|
Example: Show the Lewis structure of the carbonate ion. The carbonate ion has the formula CO32-. It consists of one central carbon atom and three oxygen atoms. The total number of valence electrons = 4 + (3 x 6) = 22. It also has a 2- charge making a total of 22 + 2 = 24 electrons. 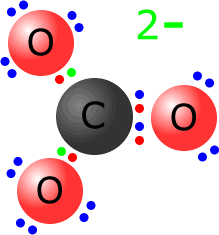
The carbon shares two pairs of electrons with one of the oxygen atoms and one pair of electrons with the other 2 oxygen atoms. This makes 8 electrons around the central carbon atom, leaving 16 electrons to be distributed over the three oxygen atoms. One of the atoms already shares four electrons, so it needs another two non-bonding pairs (4 electrons). The other two oxygen atoms need three non-bonding pairs (6 electrons). Once again, the extra electrons are shown in green, but are indistinguishable from the others. |


