|
In the previous section we looked at how the electrons organise themselves around an atom to minimise repulsions. Now, by using the Lewis structures, we can predict the shapes of molecules and ions. |
.gif)
|
Two electron domains (charge centres) always adopt a linear orientation to minimise repulsion between the negative charges.
|
In beryllium chloride the beryllium atom has only two electron domains. It uses both of them to bond the two chlorine atoms. 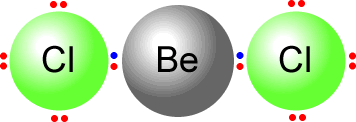
|
When the central atom has three electron domains the electronic shape adopted is trigonal planar. The actual shape of the molecule depends on the number of attached atoms.
- Three attached atoms - trigonal planar
- Two attached atoms - angular
|
The boron trifluoride molecule has the central boron atom with three regions of electron density (electron domains). These adopt a trigonal planar orientation. 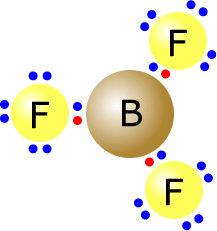
|
Three electron domains, but only two attached atoms produces an angular molecule
|
The sulfur dioxide molecule has the central sulfur atom with three electron domains. 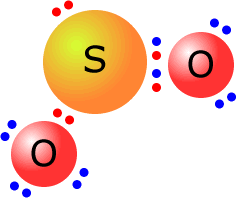
These adopt a trigonal planar orientation. Only two out of the three electron domains are used to bond oxygen atoms. The molecular shape is angular. O-S-O bond angle = 119º. The deviation from the expected bond angle of 120º is due to slightly different degrees of repulsion between the sulfur-oxygen bonding electrons and the lone pair on the sulfur atom. |
Molecules with four electron domains are very common, as four pairs of electrons make up a full octet of electrons. The domains adopt a tetrahedral orientation, but the molecular shape depends on the number of atoms or groups attached to the central atom.
| Number of attached atoms or groups | Molecular shape |
| 4 | tetrahedral |
| 3 | trigonal pyramidal |
| 2 | angular (bent) |
The bond angles that are finally produced depends on the inter-electron repulsion between the bonding pairs of electrons and the non-bonding, or lone pairs. This is best understood using some examples.
The methane molecule - VSEPR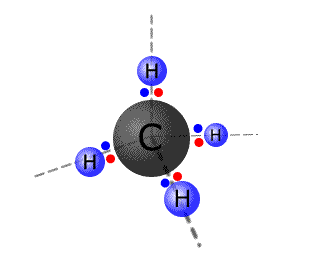
- The carbon atom has four pairs of electrons. They are arranged in a tetrahedral arrangement.
- All four of the pairs of electrons are used in bonding
- The molecular shape is tetrahedral.
- All of the H-C-H bond angles are 109.5º

