|
Molecular orbital theory is a more sophisticated and holistic view of covalent bonding that builds upon the previous theories of linear combination of atomic orbitals in conjuction with delocalisation, resonance and hybridisation. |
|
Molecular orbitals
Atoms and molecules are the physical manifestations of the stable structures that exist in our universe. An atom is an arrangement of charged particles which, except for the noble gases, cannot exist on its own for very long.
Atoms join up to form either metallic structures, molecules or ionic structures. Effectively, a molecule is just an arrangement of charged particles which is particulary stable. It is essentially a multi-centre noble gas type atom.
In the same way that atoms have orbitals in which to house their electrons, so do molecules. The shape and orientation of the atomic orbitals, as discovered by Ernst Schroedinger, was based on the energies and interactions between the positive and negative particles.
It is a perfectly logical assumption that molecules have orbitals of their own, with distinct shapes and orientations depending on the multiple nuclear centres and their interaction with the electronic charges.
These molecular orbitals are constructed according to certain rules.
- 1 The sum of the atomic orbitals of the constituent atoms must equal the sum of the molecular orbitals.
- 2 The sum of the energies of the molecular or orbitals must equal the sum of the energies of the atomic orbitals
- 3 Only occupied orbitals contribute to the energy sum.
- 4 Orbitals between nuclear centres contribute to bonding (bonding orbitals).
- 5 Orbitals not between nuclear centres do not contribute to bonding (anti-bonding orbitals).
- 6 For every bonding orbital there must be a corresponding anti-bonding orbital.
Sigma bonds
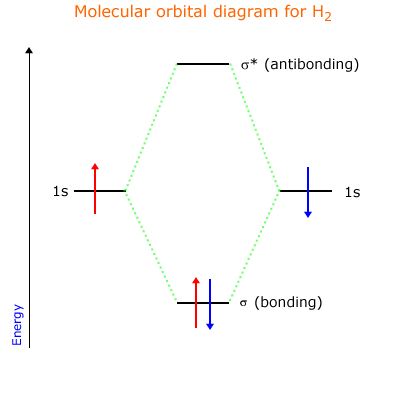
A sigma bond is a molecular orbital formed by the direct orbital overlap along an axis of two appropriately shaped orbitals, for example 's' and 'p' orbitals, or 's' and 's' orbitals.
The result is a region of electron density (electron domain) that resides between two nuclear centres. There is also a sigma antibonding orbital formed (rule 1 above) that does not contribute to bonding. This can be shown using the simplest example, the hydrogen molecule.
These are molecular orbitals formed by lateral overlap of parallel orbitals (usually 'p') resulting in regions of electron density above and below the axis that joins the two nuclear centres.
Once again, the formation of a pi molecular orbital is accompanied by the formation of a pi* antibonding orbital.

