|
As discussed in section 3.11, matter is built up with one of five structures. This section deals with the approach used when discussion the particles involved in matter according to its structure. Syllabus referenceStructure 1.4.1 - The mole (mol) is the SI unit of amount of substance. One mole contains exactly the number of elementary entities given by the Avogadro constant.
Guidance
Tools and links |
All of the atoms are held together by weak forces only. The substances are always gases at room temperature, liquifying and solidifying at temperatures far below zero celsius (0ºC). As the only known cases of simple atomic structure are the noble gases (Group 0), they are of little interest as regards moles calculations.
 |
|
1 mole of any noble gas contains 1 mole of atoms = 6.02 x 1023 atoms of that gas.
1 mole of a simple molecular substance contains 1 mole of molecules of that substance = 6 x 1023 molecules
In the case of water, where 1 molecule is made up of two hydrogen atoms and 1 oxygen atom bonded together, it is clear that each molecule contains a total of three atoms. We can talk about these atoms as if they were not chemically bonded.
 |
|
It is valid to say that 1 mole of water contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms.
Notice that it is important to specify that you are talking about atoms. To say that 1 mole of water contains 1 mole of oxygen is incorrect. 1 mole of oxygen implies 1 mole of oxygen 02 molecules as this is the usual state of oxygen under standard conditions.
|
Example: The compound sucrose has a formula C12H22O11. Calculate the number of atoms of oxygen in 1 mole of sucrose 1 mole of glucose contains 6.02 x 1023 sucrose molecules and 1 molecule of sucrose contains 11 oxygen atoms Therefore 6.02 x 1023 sucrose molecules contain 6.02 x 1023 x 11 oxygen atoms Answer = 6.62 x 1024 oxygen atoms |
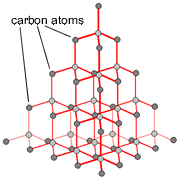
These structures have a giant lattice in which the atoms are all covalently bonded together, effectively making the entire structure 1 molecule. The atoms may be all the same, in the case of a giant molecular element such as diamond, or they may be different as in silicon dioxide.
 |
 |
|
Diamond
|
Silicon dioxide
|
The elemental giant structures may be discussed as if the atoms were not bonded together, eg 1 mole of carbon contains 6 x 1023 carbon atoms.
In the case of the giant covalent compounds the simplest ratio of atoms withn the structure is considered to be the "formula unit". 1 mole of silicon dioxide means 1 mole of silicon dioxide formula units.
The simplest ratio of silicon to oxygen within the giant structure is 1 silicon atom to every 2 oxygen atoms; the formula unit is SiO2. 1 mole of silicon dioxide contains 1 mole of silicon atoms and 2 moles of oxygen atoms.
|
Example: The compound silicon dioxide has a formula SiO2. Calculate the number of atoms of oxygen in 0.2 moles of silicon dioxide. The formula unit is SiO2, therefore 1 mole of silicon dioxide contains 2 moles of oxygen atoms. Therefore 1 mole of silicon dioxide contains 0.2 x 2 x 6.02 x 1023 oxygen atoms Answer = 2.41 x 1023 oxygen atoms |
Ionic compounds are giant structures comprising a lattice of oppositely charged ions. The simplest ratio of the ions is called the formula unit. 1 mole of an ionic compound is understood to contain 1 mole of formula units of that substance.
 |
|
|
Example: The compound calcium chloride is a giant lattice of calcium and chloride ions. The simplest ratio of ions within the structure is 1 calcium ion for every 2 chloride ions giving a formula unit of CaCl2 1 mole of calcium chloride contains 1 mole of calcium ions = 6.02 x 1023 calcium ions 1 mole of calcium chloride contains 2 moles of chloride ions = 1.2 x 1024 chloride ions |
Metallic structures comprise a giant structure of metal atoms within which the valence electrons are delocalised. In terms of discussing the number of moles the structure can be considered to be made up of associated atoms.
 |
|
Therefore 1 mole of any metal contains 6 x 1023 metal atoms.

