|
Diffusion is the movement of particles from a region of high concentration to a region of low concentration. |
 |
Graham's law
The rate of diffusion is dependent on the density of the gas and hence its relative molecular mass. The smaller the relative molecular mass the faster the rate of diffusion.
Logically this makes sense. It would be easier for a small ch¡ld to find his way through a crowd of adults than for a very large man to do the same.
The actual dependency follows the relationship:
For example hydrogen gas, Mr = 2, diffuses fastest, as the term 1/Mr is the largest.
A comparison of ammonia (Mr = 17 and hydrogen chloride (Mr = 36.5) shows that the relative rates of diffusion are √1/17 for ammonia and √1/36.5 for hydrogen chloride, respectively.
This approximates to a relative diffusion rate of 1/4 for ammonia to 1/6 for hydrogen chloride, i.e. ammonia diffuses 3/2 times faster than hydrogen chloride (obtained by multiplying both fractions by 6). You would expect ammonia to diffuse 3/2 times the distance diffused by HCl over the same time period.
|
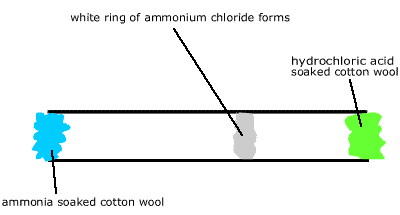
Demonstration Experiment Place wads of cotton wool in both ends of a horizontally clamped 1 m long glass tube. Soak one wad with concentrated HCl and the other with concentrated ammonia solution and observe. After a while a white ring of ammonium chloride forms in the tube. The ring appears 60cm from the ammonia soaked wad and 40cm from the HCl soaked wad. |
 |
Calculations involving Graham's law are not required for standard or higher level, but are required for option C (our thanks to Jeffrey Frankel for pointing this out).

