|
Chemical compounds are groups of atoms or ions held together by chemical bonds. The definition of a chemical reaction is 'a process in which a new substance is formed'. For a chemical reaction to occur, these bonds must be broken before others can form. |
|
Bond dissociation enthalpy
This is the energy required to break one mole of specific bonds in a specific molecule. It only applies to one particular type of bond in a unique molecule.
|
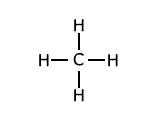
Example: Methane has the structure:
The average of the four bond dissociation enthalpies above is: (435 + 444 + 444 + 339)/4 = +415.5 kJ This average value is called the average bond enthalpy, or bond enthalpy term for C-H. |
Bond dissociation enthalpies between non-identical atoms are of limited use, as they do not apply to any other molecule.
However, they are useful in the case of specific dissociation of molecules. For example the process:
| Cl2(g) → 2Cl(g) |
Represents the dissociation enthalpy of chlorine gas. We cannot measure this value in any other molecule (we wouldn't want to!). The value is useful for construction of Born Haber cycles, or other energetics calculations.
REM It is important to be able to distinguish between bond dissociation enthalpy and average bond enthalpy, which is discussed in the next section. It is often asked for in exams.
| Bond | Bond dissociation enthalpy / kJ mol-1 |
| H-H | +432 |
| O=O | +494 |
| N≡N | +942 |
| F-F | +155 |
| Cl-Cl | +242 |
| Br-Br | +190 |
| I-I | +148 |
| Ref: CRC Handbook of chemistry and physics - Edition 44 | |