|
Acid rain is a common name for the deposition of acidic material from the atmosphere. This may be either wet deposition of acid in precipitation (rain, snow, or fog), or dry deposition of acidic material on dust, smoke, or small, microscopic particles in the air. Acid deposition was a major problem in Europe and the USA in the last few decades of the 20th century. Strong emission control laws have greatly reduced the problem in these areas. However, it continues to be a major issue in regions downwind of heavy industry. |
|
Why is rain acidic?
The atmosphere contains the soluble gas carbon dioxide, which dissolves in the rain giving a solution with pH 5.6.
CO2 + H2O ![]() H2CO3
H2CO3
The carbonic acid formed is weakly acidic by dissociation:
H2CO3 ![]() HCO3- + H+
HCO3- + H+
The term "acid depostion" refers to precipitation, rain, hail, snow etc. with a pH lower than 5.6. It is caused by dissolved gases in the water making up the precipitation. These gases react with water forming acids.
Acidic pollution
The English chemist Robert Angus Smith is generally credited with coining the phrase 'acid rain' in 1872 to describe the increased acidity of the rain in British industrial centers (such as Manchester), which was apparently caused by the unbridled excesses of the early Industrial Revolution.
Typical pH values for rain in the continental United States now range from 4 to 4.5, with values as low as 2.0 reported for areas such as Los Angeles. Even “normal” rain is now as acidic as tomato juice or black coffee.These are mostly oxides of sulfur and nitrogen that are present in the air due to natural or anthropogenic (human) activity. All of these non-metal oxides dissolve in water forming acids.
The nitrogen oxides are described by the general formula NOx.
The sulfur oxides are either sulfur(IV) oxide or sulfur(VI) oxide.
Nitrogen oxides, NOx
Natural sources
Nitrogen is present in the air at a percentage of about 80%. Nitrogen is a very stable gas and reacts only with difficulty. However, the temperatures attained during electrical discharges cause nitrogen to combine with the oxygen in the air producing nitrogen monoxide, NO.
N2 + O2 → 2NO
Nitrogen(II) oxide (nitrogen monoxide) is a very reactive gas that combines directly with oxygen in the air forming nitrogen(IV) oxide (nitrogen dioxide):
2NO + O2 → 2NO2
Nitrogen dioxide is a red brown gas which dissolves easily in water forming an acidic solution:
H2O + 2NO2 → HNO2 + HNO3
This is a disproportionation reaction, with the nitrogen in oxidation state +4 being simultaneously oxidised to nitrogen(V) and reduced to nitrogen(III).
The nitric(III) acid formed in the equation above may also react further with oxygen in the air to produce more nitric(V) acid.
2HNO2 + O2 → 2HNO3
Ultimately then, most of the nitrogen oxides in the atmosphere end up as nitric acid, a very strong acid.
Human activity
 The
20th century has been the century of the motor car. The development of the
internal combustion engine made individual motorised transport affordable
for a large percentage of the developed world.
The
20th century has been the century of the motor car. The development of the
internal combustion engine made individual motorised transport affordable
for a large percentage of the developed world.
However, the technology relies on the production of an electrical spark that explodes a mixture of fuel and air. This spark is effectively a tiny electrical discharge and generates a high local temperature, enough to combine some of the nitrogen and oxygen in the air mixture.
The consequence is that the exhaust emissions of internal combustion engines contain nitrogen oxides. In cities, where the number of cars is huge, the pollution caused by nitrogen oxide emissions is visible in the brown haze that often sits above the cities during periods of calm weather.
In the USA, between 1900 and 1970, NOx emissions increased 690 percentref and this trend would have continued but for the US government passing the clean air act in 1963.
In general, however, the problem still exists in developing countries.
Sulfur oxides
Natural sources

Large amounts of sulfur dioxide have always been released into the atmosphere by natural sources, such as volcanic activity, forest fires, and the microbial decay of organic materials.
Emission rates of SO2 from an active volcano range from less than 20 tonnes per day to more than 10 million tonnes per day according to the style of volcanic activity and type and volume of magma involved.
For example, the large explosive eruption of Mount Pinatubo on 15 June 1991 expelled 3-5 km3 of dacite magma and injected about 20 million metric tons of SO2 into the stratosphere. ref
However, for most of Earth’s recorded history the natural cycling of sulfur from the atmosphere into oceans and rocks has kept the acidity of rain and snow in balance.
Human activity
Sulfur dioxide emissions increased enormously during the 20th century with the increased use of fossil fuels. Fossil fuels are derived from living systems, which have proteins containing sulfur. This means that all fossil fuels have an appreciable sulfur content.
Many coals contain 5-6% iron(II) sulfide, FeS, and crude ion is typically 0.5% sulfur.
Burning these fuels releases sulfur dioxide into the atmosphere.
S + O2 → SO2
Effects of acid deposition
Sulfur dioxide pollution
Sulfur dioxide dissolves readily in water forming sulfuric(IV) acid, which is then oxidised in the presence of oxygen (in the air) to sulfuric acid:
2SO2 + H2O → H2SO3
2H2SO3 + O2 → 2H2SO4
Sulfuric acid is a strong acid which attacks buildings and metals such as iron. If it enters the living environment it decreases the pH levels and kills plants and aquatic animals.
H2SO4 + Fe → Fe2SO4 + H2
Sulfuric acid also attacks the naturally occuring carbonate ions in rocks such as dolomite (magnesium carbonate) and limestone and marble (calcium carbonate).
H2SO4 + MgCO3 → MgSO4 + H2O + CO2
NOx pollution
The natural balance of the earth makes use of the nitric acid in the air by reacting it with basic rocks such as limestone (calcium carbonate and dolomite (magnesium carbonate) forming nitrates, which are needed by plants for growth.
Nitrogen oxides also produce acids when inhaled and cause bronchitis and pneumonia.
Nitric acid is an oxidising acid and can react with unreactive metals such as copper and lead:
8HNO3 + 3Cu → 3Cu(NO3)2 + 2NO + 4H2O
Notice that one of the products is nitrogen monoxide, NO, which then goes on to react with oxygen in the air as outlined above.
Nitric acid is a strong acid that attacks carbonate based rocks such as dolomite (magnesium carbonate) and limestone (calcium carbonate):
2HNO3 + CaCO3 → Ca(NO3)2 + H2O + CO2
Acid deposition in general
Acid deposition can react with limestone/marble based buildings, causing erosion, corrosion and decay.
The biological effects of acid rain are more complex. Organisms such as fish can maintain their internal pH (7-8) in water that has a pH in the range of 6.5–8.5. If the external pH is too low, however, many aquatic organisms can no longer maintain their internal pH, so they die.
A pH of 4 or lower is fatal for virtually all fish, most invertebrate animals, and many microorganisms. As a result of acid rain, the pH of some lakes in Europe and the United States has dropped below 4.
|
Case study In 2001 the precipitation that fell on the Adirondack Mountain lakes had, on average, a pH of 4.5. For many lakes with little buffering capacity, acid precipitation translates into more acidic water, until eventually all of the fish die. About 27% of the 2,800 lakes and ponds in the Adirondacks have a pH of 5.0 or less all year round. Many more dip below 5.0 at certain times of the year, typically during snowmelt. The Environmental Protection Agency estimates that by 2040, 43% of lakes in the Adirondacks will have pH below 5.0 year-round and therefore be deadly to fish.ref |
These locations do not contain large concentrations of industry, but New York lies downwind of the industrial Midwest, and Scandinavia is downwind of the most industrialized regions of western Europe. Both regions appear to have borne the brunt of the pollution produced by their upwind neighbors.
One possible way to counter the effects of acid rain in isolated lakes is by adding large quantities of finely ground limestone, which neutralizes the acid:
2HNO3 + CaCO3 → Ca(NO3)2 + H2O + CO2

A second major way in which acid rain can cause biological damage is less direct.
Trees and many other plants are sensitive to the presence of aluminum and other metals in groundwater. Under normal circumstances, aluminum hydroxide [Al(OH)3], which is present in some soils, is insoluble. At lower pH values, however, Al(OH)3 dissolves via the following reaction:
Al(OH)3 + 3H+ → Al3+ + 3H2O
The result is increased levels of Al3+ ions in groundwater.
Al3+ ions are toxic to plants and high concentrations can affect plant growth. Acid rain can also weaken the leaves and roots of plants so much that the plants are unable to withstand other stresses.
The combination of the two effects can cause significant damage to established forests, such as the Black Forest in Germany and the forests of the northeastern United States and Canada and other countries.
Reducing acid deposition
There is very little that can be done about the natural sources of acid deposition, and in any case the earth is naturally balanced to cope with these emissions. The problem has arisen because of the contributions from anthropogenic sources. Any attempt to tackle the issue must look at these.
Nitrogen oxides, NOx

With nitrogen oxides the main source is car engines. Cars can be fitted with catalytic converters that react the nitrogen oxides formed in the engine with carbon monoxide giving nitrogen and carbon dioxide, both relatively harmless gases. The catalyst used is usually a combination of the metals rhodium, platinum and palladium (4-9g) supported on an inert ceramic honeycomb structure.
A three-way catalytic converter has three simultaneous functions:
1 Reduction of nitrogen oxides into elemental nitrogen and oxygen:
2NO → O2 + N2
2 Oxidation of carbon monoxide to carbon dioxide:
2CO + O2 → 2CO2
3 They also oxidise hydrocarbons into carbon dioxide and water:
CxH(2x+2) + [0.5(3x+1)]O2 → xCO2 + (x+1)H2O
A catalytic converter can be up to 98% efficient, which of course means that at least 2% of the pollutant gases escape. There is also the problem of the extra cost of a catalytic converter making it undesirable in poorer countries.
Sulfur dioxide, SO2
There are two approaches to reduction of sulfur dioxide pollution from burning fossil fuels.
1 Remove the sulfur from the fossil fuels before combustion (pre-combustion method)
2 Remove the sulfur dioxide from the emissions produced by the combustion (post-combustion method)
Pre-combustion method
This is usually carried out using hydrogen gas during the refining process (hydrodesufurisation or hydrotreatment).
The hydrogen gas reacts with the sulfur in the crude oil producing hydrogen sulfide gas that can be separated and lead to sulfur recovery units where it is oxidised to sulfur. This produces vast amounts of sulfur that can be used in the rubber industry or simply stored.

Post-combustion method
The gases formed by combustion of fossil fuels is passed through alkaline 'scrubbers' that remove any acidic gases. These scrubbers contain calcium oxide, formed by roasting calcium carbonate, and water spray that combines with the sulfur dioxide making calcium sulfite dihydrate, CaSO3.2H2O.
CaO + SO2 → CaSO3
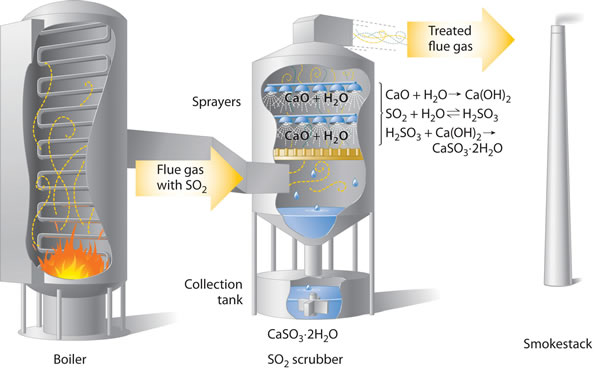 Removing
SO2 from the gases prevents its conversion to acid rain. Scrubbing
systems are now commonly used to minimize the environmental effects of large-scale
fossil fuel combustion.
Removing
SO2 from the gases prevents its conversion to acid rain. Scrubbing
systems are now commonly used to minimize the environmental effects of large-scale
fossil fuel combustion.


