|
'Iso' derives from the Greek word 'isos', meaning 'equal'. 'Mer' derives from the Greek word 'meros', meaning 'parts'. Isomers = having equal parts. Isomers contain exactly the same atoms in number and quantity, but with a different arrangement. |
|
Structural isomerism
Isomerism means molecules that have the same molecular formula, but differ in the arrangement of their atoms relative to one another, either structurally or spatially.
Structural isomers have the same molecular formula, but differ in their arrangement of atoms. They may have a different arrangement of the same structural groups or they may have a different arrangement of atoms, giving rise to different functional groups.
Positional isomerism
This may be caused by branching, or positioning the functional groups on different carbon atoms in the main carbon chain.
|
methylbutane
|
dimethylpropane
|
 |
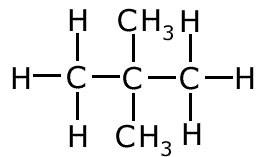 |
The two molecules above both have the molecular formula C5H12, but have a different arrangement of carbon atoms. In the first case the longest chain is four carbon atoms long and in the second case it is three carbon atoms long. The two molecules are identical in molecular formula and belong to the same homologous series, but have different structures.
|
butan-1-ol
|
butan-2-ol
|
 |
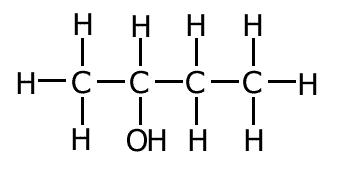 |
In the two molecules above, the only difference is the position of the functional group. This is an example of positional isomerism.
Functional group isomerism
Functional group isomers have their atoms arranged differently in the isomers giving rise to different functional groups.
|
ethenol
|
ethanal
|
 |
 |
In this example the molecule ethenol has two functional groups, a double bond and an alcohol group. In the ethanal molecule there is only one functional group, the aldehyde (alkanal) group. Changing the arrangement of the atoms has changed the nature of the functional groups.

