|
Solubility refers to the dispersion of a solute in a solvent to make a homogeneous solution. |
|
Dissolution
Water is the most common solvent, consequently solubility is usually described in terms of solution in water at room temperature.
The process of solubility is not quite as straightforward as it seems. Firstly the solid structure must be broken apart, then the water molecules must bond to the particles that are in the solution.
- Breaking a solid structure apart requires energy (the bonding between the particles in the solid must be overcome).
- Bonds formed by the solvent (usually water) and the particles from the solid release energy.
However, there is also the contribution of entropy to the process. Dissolution usually leads to an increase in disorder and hence an increase in entropy. Because of the contribution from the entropy, the overall energy change may be exothermic or endothermic. This makes prediction of solubility more complicated when viewed from a thermodynamic (energetic) perspective.
Thermodynamically for a substance to be soluble the Gibbs Free Energy change for the process must be negative. Gibbs Free Energy change is related to the enthalpy and entropy changes at a specific temperature by the equation:
![]()
Clearly, the temperature at which the solubility is measured has a bearing on the result. Most substances increase solubility as the temperature is increased.
Two factors that make prediction of solubility a little easier in organic chemistry are:
- Hydrocarbon chains are non-polar and do not form bonds with water molecules. They are said to be hydrophobic (water hating).
- Some functional groups form hydrogen bonds with water, allowing the molecule to dissolve. These groups are said to be hydrophilic (water loving)
Hydrocarbons
The hydrocarbon chain is hydrophobic, consequently alkanes, alkenes and alkynes do not dissolve in water. They dissolve in non-polar solvents, such as benzene and tetrachloromethane.
Halogenoalkanes
Halogenoalkanes are polar, but only form weak bonds with water molecules, not enough to confer solubility. Lower members of the halgenoalkanes are slightly soluble and this is also affected by the nature of the halogenoalkane, with tertiary halogenoalkanes being more soluble than secondary, which in turn are more soluble than primary halogenoalkanes.
Alcohols
The alcohol, -OH, group is polar and able to form hydrogen bonds with water molecules. This means that alcohols are soluble. However, as the relative molecular mass increases, ascending the homologous series, the hydrophobic hydrocarbon chain begins to hinder the solubility process.
The result is that methanol and ethanol are miscible in all proportions with water, but the solubility decreases on ascending the series.
The alcohol group hydrogen bonds very easily with water. This means that molecules with more than one -OH group are often very soluble even though they may be large. This is the case with the sugars, such as glucose.
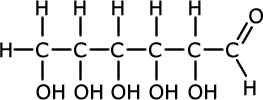 |
|
Glucose
|
Carboxylic acids
The -COOH group forms strong hydrogen bonds with water and dissociates partially in solution releasing free hydrogen ions. This makes carboxylic acids soluble. Once again, large hydrocarbon chains reduce the solubility for carboxylic acids with high relative molecular masses.
The benzene carboxylic acid is only slightly soluble.
Aldehydes and ketones
Both of these contain a highly polar carbonyl group -CO, that forms reasonably strong bonds with water molecules. This means that the lower members of the aldehydes and ketones are miscible with water in all proportions but the solubility decreases as the hydrocarbon chain length increases.
Nitrogen containing compounds
Nitrogen is less electronegative than oxygen, but has a high enough electronegative value to produce hydrogen bonding when hydrogen atoms are directly attached to the nitrogen.
Ammonia, for instance, is extremely soluble in water; the lone pair available on the nitrogen atom attacting hydrogen atoms on neighbouring ammonia molecules. Amines are likewise soluble.
Amides also have hydrogen atoms attached directly to the nitrogen atoms and the added advantage of electronegative oxygen atoms with available lone pairs of electrons. Hence, amides are even more soluble.
As the carbon chain increases with both amines and amides, the solubility decreases proportionally.
Summary
|
Homologous series
|
Solubility
|
| alkanes, alkenes | Insoluble |
| halogenoalkanes, amines (more so than halolkanes) | Slightly soluble |
| alcohols, carboxylic acids, aldehydes, ketones, amides | Soluble |
Increasing the length of the hydrocarbon chain reduces the solubility.

