|
Alkenes are hydrocarbons that contain an alkenyl functional group. They are manufactured industrially by cracking hydrocarbons and are much more useful than alkanes chemically, as the double bond makes them reactive towards many other species. Syllabus referenceReactivity 3.2.11 - Reduction of unsaturated compounds by the addition of hydrogen lowers the degree of unsaturation.
Guidance Tools and links
|
Structural features
Alkenes are organic compounds that contain a double carbon-carbon (alkenyl) bond. They are said to be 'unsaturated' in that they can add more hydrogen atoms to the hydrocarbon skeleton.

The double carbon-carbon bond is a region of high electron density, with one pair of electrons in a 'pi' orbital, which can be attacked by reagents containing a positive or partial positive charge. This makes alkenes reactive and useful as building blocks for organic synthesis.
Naming alkenes
The position of the double bond is numbered using the carbon with the smallest number in the chain. If there is branching the chain is chosen that includes the double bond.
|
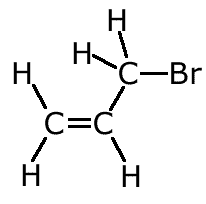
Example: Name the following alkene:
The longest chain with the double bond has three carbon atoms. The chain is numbered to keep the double bond with the lowest number. The bromine, therefore is on carbon number 3. Hence 3-bromopropene |
Electrophilic addition reactions
Alkenes can form addition products with other molecules by 'opening' the double bond and using the electrons to form bonds at each carbon atom. This is known as addition.
The process is stimulated by electrophilic attack by the reagent, and for this reason is called electrophilic addition.
Alkenes react with halogens making disubstituted halogenoalkanes
|
ethene + bromine → 1,2-dibromoethane C2H4 + Br2 → C2H4Br2 |
Alkenes react with interhalogens making disubstituted halogenoalkanes
|
ethene + iodine monochloride → 1-chloro-2-iodoethane C2H4 + ICl → CH2ClCH2I |
Alkenes react with hydrogen halides making halogenoalkanes:
|
ethene + hydrogen bromide → bromoethane C2H4 + HBr → C2H5Br |
Alkenes react with steam in the presence of a catalyst making alcohols:
|
ethene + steam → ethanol C2H4 + H2O → C2H5OH |
Alkenes react with hydrogen in the presence of a Ni catalyst at 150ºC making alkanes:
|
ethene + hydrogen → ethane C2H4 + H2→ C2H6 |
Although it may seem pointless making an unreactive alkane from its useful alkane, this reaction is important in synthesis, as it allows control of the degree of unsaturation of long chain compounds that have several double bonds, such as the vegetable oils.
Changing double bonds to single bonds, via hydrogenation, increases the melting point of a vegetable oil, an essential step in the production of margarine.
The mechanism of electrophilic addition
An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids. Deduction of the mechanism of the electrophilic addition reactions of alkenes with halogens/interhalogens and hydrogen halides.
Step 1: Attack by the electrophile - the pair of electrons in the pi orbital of the alkenyl group moves to attach the partially positive side of the polarised electrophile forming a carbo-cation (positive) intermediate and breaking the bond in the electrophile heterolytically.
Step 2: The negative ion remaining from the heterolytic fission of the electrophile then attacks the carbon atom holding the positive charge on the intermediate.
Mechanism - electrophilic addition
Addition to asymmetric alkenes
Asymmetric alkenes have different groups on either side of the double bond. For example propene, CH2CHCH3.

When electrophilic addition occurs with an asymmetric alkene there is a choice of possible products.
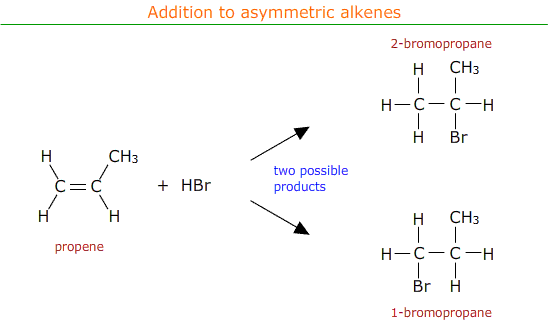
In practise both of the possible products are formed, but in different amounts. There is a major product with a higher percentage yield and a minor product with a lower percentage yield.
Markovnikov's rule can be applied to predict the major product.
Markovnikov's rule
Markovnikov's rule says that the hydrogen atom from the electrophile (HBr in this case) preferentially adds to the carbon atom of the double bond that already has the most hydrogen atoms attached.
It may be seen that in the above example the major product is 2-bromopropane.
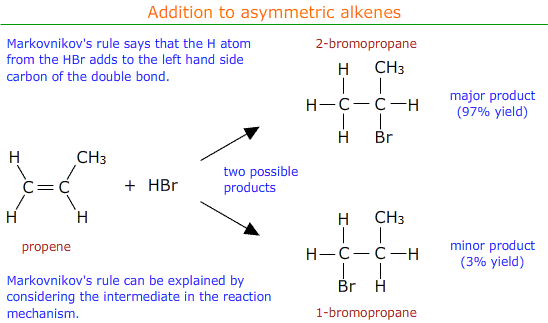
The major and minor products can be explained in terms of the relative stability of the two possible carbo-cation intermediates formed in the reaction mechanism.

Polymerisation
Alkenes can polymerise in the presence of suitable catalysts. Originally this was carried out using high pressure and an oxygen catalyst, but nowadays there are more efficient catalysts containing titanium compounds that can make better polymeric products (Zeigler-Natta catalysts).
|
C2H4 → -[C2H4]n- ethene → poly(ethene) |
By changing the substituents around the double bond, a large variety of different polymers can be produced, each one with different properties.
|
C6H5CHCH2 → -[C6H5CHCH2]n- phenylethene (styrene) → poly(styrene) |