|
General properties and condensation reactions of carboxylic acids are covered in this section. Syllabus referenceReactivity 3.2.10 - Functional groups in organic compounds may undergo reduction.
Guidance
Tools and links
|
Structure
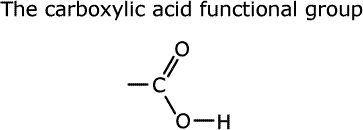
Carboxylic acids have hydrogen, or an alkyl group attached to the -COOH functional group.
|
Formula
|
Name
|
|
HCOOH
|
methanoic acid
|
|
CH3COOH
|
ethanoic acid
|
|
C2H5COOH
|
propanoic acid
|
|
C3H7COOH
|
butanoic acid
|
This functional group has a carbon-oxygen double bond and a carbon-oxygen single bond, with this oxygen attached to an acidic hydrogen.
Physical properties
The lower members of the homologous series are high boiling point liquids. This is due to the high degree of hydrogen bonding that holds the carboxylic acid molecules together. There is evidence that the acids exist as dimers even in the vapour state.
High relative molecular mass carboxylic acids are solids for the same reasons, i.e. strong intermolecular forces.
Chemical properties
Carboxylic acids are weak acids. They undergo reactions typical of acids, they react with alcohols to form esters, the OH group can undergo substitution reactions with powerful reagents and the carboxyl group can be reduced by powerful reducing agents such as lithium aluminium hydride (lithium tetrahydroaluminate) to aldehydes and, subsequently, primary alcohols.
Summary
- Acid reactions
- Esterification
- Reduction
- Substitution of the OH group
Acidic properties
The -COOH functional group dissociates in aqueous solution according to the equation:
|
CH3COOH
|
 |
H+(aq) + CH3COO-(aq)
|
This means that carboxylic acids undergo all of the usual reactions of acids, if a little more slowly.
- With bases making salt and water
- With carbonates making salt + water + carbon dioxide
- With active metals making salt + hydrogen
The acidic nature of carboxylic acids can be used as a test. The suspected carboxylic acid is added to a solution of sodium hydrogen carbonate and if bubbles of gas are seen then this is taken as a positive identification.
Acid strength
The strength of a carboxylic acid depends on two factors:
- The ease with which the O-H bond is broken
- The relative stability of the ion formed
The first factor is affected by electron withdrawing groups attached to the carbon chain. These draw electron density away from the carboxylic acid group, weakening the O-H bond, making loss of the hydrogen ion easier. The consequence is greater acidity. Electron inducing (pushing) groups such as the alkyl group have the reverse effect.
Example
|
|
The acid strength is given by the acid equilibrium constant, Ka, or its pKa value. For the equilibrium:
|
CH3COOH
|
 |
H+(aq) + CH3COO-(aq)
|
The acid dissociation constant is given by:
![]()
The stronger an acid is the greater its Ka value and the smaller its pKa value.
|
acid
|
Ka
|
pKa
|
| ethanoic acid | 1.78 x 10-5 |
4.75
|
| chloroethanoic acid | 1.38 x 10-3 |
1.86
|
| dichloroethanoic acid | 2.20 x 10-1 |
0.66
|
| trichloroethanoic acid | 5.10 x 10-2 |
1.29
|
Notice that the trichloroethanoic acid is less acidic than would be thought from consideration of the electron withdrawing effect of the chlorine atoms alone. There is clearly another factor to be considered.
Esterification
Esterification is the reaction between a carboxylic acid and an alcohol forming an ester and water.
|
CH3COOH + CH3OH
|
 |
CH3COOCH3 + H2O
|
The reaction is an equilibrium and it is helped by the addition of concentrated sulfuric acid which both acts as a catalyst, speeding up the attainment of equilibrium, as well as a dehydrating agent, mopping up the water as it is formed and pulling the equilibrium to the right hand side (according to Le Chatelier's principle).
Naming the product
The original carboxylic acid provides the ending of the name → carboxylate
The original alcohol gives the beginning of the ester's name → alkyl
|
Example: Name the ester formed by reaction between methanoic acid and ethanol Methanoic acid → methanoates Ethanol → ethyl Therefore the name of the ester = ethyl methanoate |
Reactions of the -OH group
Certain reagents cause removal of the -OH atoms from the -COOH group, substituting them for another atom or group.
eg. Chlorination using PCl5
|
CH3COOH + PCl5
|
 |
CH3COCl + POCl3 + HCl
|
Not required at IB
Reduction
Carboxylic acids can be reduced to aldehydes, and further to primary alcohols, by strong reducing agent such as lithium aluminium hydride (lithium tetrahydroaluminate). This reagent is used in ethoxyethane (diethyl ether) as it is very water sensitive. The complex formed is then hydrolysed by dilute acid to the final product.
|
CH3COOH + [LiAlH4] CH3CHO + [LiAlH4] |

