|
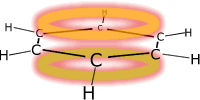
Benzene is the simplest aromatic compound. It has a delocalised pi system around a ring of carbon atoms making the molecule electron rich. This makes benzene susceptible to attack by electrophiles. |
|
Nitration of benzene
Benzene rings are electron rich and as such are attractive to electrophiles. One such electrophile is the nitronium ion, NO2+, which can be formed 'in-situ' by the reaction between concentrated sulfuric acid and concentrated nitric acid. This is known as nitration as one of the benzene ring's hydrogen atoms is substituted by the NO2 (nitro) group.
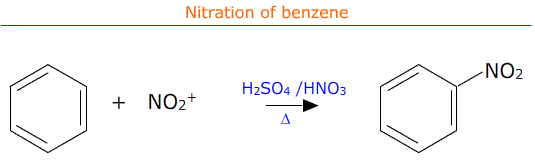
The actual equation for the reaction may be written as:
C6H6 + HNO3 → C6H5NO2 + H2O
Mechanism
The mechanism of nitration of benzene is called electrophilic substitution as it involves attack by an electrophile and the overall change is one of substitution.
The first stage is formation of the nitronium ion 'in-situ' by protonation of nitric acid by hydrogen ions from the sulfuric acid.
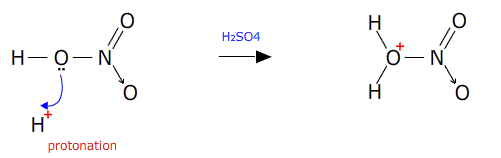
The intermediate is then dehydrated in the presence of concentrated sulfuric acid (a good dehydrating agent).
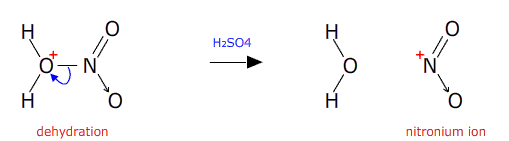
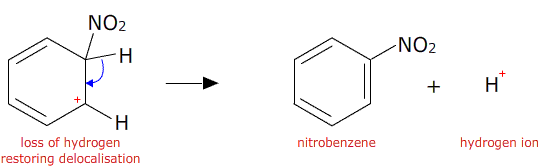
The nitronium ion then attacks the benzene ring. An electron pair from the delocalised pi orbital reaches out and attaches to the nitronium ion. This temporarily breaks the delocalisation.

A hydrogen ion is then lost from the benzene ring and the electron pair is returned to the ring restoring the delocalisation.
Benzene and its derivatives undergo many other types of electrophilic substitution reactions.

