Standard level
Chemical compounds are groups of atoms or ions held together by chemical bonds. The definition of a chemical reaction is 'a process in which a new substance is formed'. For a chemical reaction to occur, these bonds must be broken before others can form.
Syllabus ref: R1.2.1Reactivity 1.2.1 - Bond-breaking absorbs and bond-forming releases energy.
- Calculate the enthalpy change of a reaction from given average bond enthalpy data.
Guidance
- Include explanation of why bond enthalpy data are average values and may differ from those measured experimentally.
- Average bond enthalpy values are given in the data booklet.
Tools and links
- Structure 2.2 - How would you expect bond enthalpy data to relate to bond length and polarity?
- Reactivity 3.4 - How does the strength of a carbon–halogen bond affect the rate of a nucleophilic substitution reaction?
Bond dissociation enthalpy
This is the energy required to break one mole of specific bonds in a specific molecule. It only applies to one particular type of bond in a unique molecule.
|
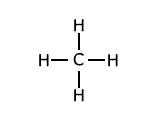
Example: Methane has the structure:
The energy change for the process: CH4 → CH3 + H ΔH = +435 kJ This is different from the energy change required for the process: CH3 → CH2 + H ΔH = +444 kJ which is different from: CH2 → CH + H ΔH = +444 kJ which is different from: CH → C + H ΔH = +339 kJ The average of the four bond dissociation enthalpies above is: (435 + 444 + 444 + 339)/4 = +415.5 kJ This average value is called the average bond enthalpy, or bond enthalpy term for C-H. |
Bond dissociation enthalpies between non-identical atoms are of limited use, as they do not apply to any other molecule.
However, they are useful in the case of specific dissociation of molecules. For example the process:
Cl2(g) → 2Cl(g)![]() ΔH = +242 kJ mol-1
ΔH = +242 kJ mol-1
Represents the dissociation enthalpy of chlorine gas. We cannot measure this value in any other molecule (we wouldn't want to!). The value is useful for construction of Born Haber cycles, or other energetics calculations.
REM It is important to be able to distinguish between bond dissociation enthalpy and average bond enthalpy, which is discussed in the next section. It is often asked for in exams.
| Bond | Bond dissociation enthalpy / kJ mol-1 |
| H-H | +432 |
| O=O | +494 |
| N≡N | +942 |
| F-F | +155 |
| Cl-Cl | +242 |
| Br-Br | +190 |
| I-I | +148 |
| Ref: CRC Handbook of chemistry and physics - Edition 44 | |
Average bond enthalpy
The average bond enthalpy term is the average amount of energy needed to break a specific type of bond, measured over a wide variety of different gaseous molecules. It is essentially the average of all of the bond dissociation enthalpies for a specific type of bond.
| Table of bond enthalpies / kJ mol-1 | |
| C-C | 348 |
| C-H | 412 |
| C-O | 360 |
| C=C | 612 |
| C=O | 743 |
| Cl-Cl | 242 |
| O-H | 463 |
| Cl-H | 431 |
| C-Cl | 338 |
| O=O | 496 |
| Ref: CRC Handbook of chemistry and physics - Edition 44 | |
Bond enthalpies are measured per mole of bonds. The units of the bond enthalpy term is kJ mol-1
Reaction enthalpy from bond energy terms
For any chemical reaction:
Reactants → products
There is a rearrangement of the bonding between the component atoms of the reactants and products. Thanks to Hess' law, we can follow an alternative route from the reactants to the products by breaking all of the bonds and then reforming the new bonds.
Breaking the reactant's bonds is an endothermic process, it requires energy. Making the product's bonds is an exothermic process. Using the alternative route via the individual atoms we can state that the enthalpy change of the reaction is the SUM of these two processes.
Enthalpy change = step 1 (bond breaking) + step 2 (bond formation)
However, forming the products releases energy, i.e. delta H is negative, giving:
Σ the reactant bond enthalpies - Σ the product bond enthalpies.
ΔHo(reaction) = ΣΔHo(bond enthalpy reactants) - ΣΔHo(bond
enthalpy products)
In reality, all of the bonds in the reactants are not broken before reforming them into the products, this is another example Hess' law; the route may be different, but the final answer must be the same.
Example: Use the bond enthalpy terms from the table above to find the enthalpy change of the following reaction:
CH4 + 2O2 → CO2 + 2H2O
Sum of the bond enthalpy terms of the reactants:
- 4 x C-H = 4 x 412 = 1648 kJ
- 2 x O=O = 2 x 496 = 992 kJ
Total = 2640 kJ
Sum of the bond enthalpy terms of the products
- 2 x C=O = 2 x 743 = 1486 kJ
- 4 x H-O = 4 x 463 = 1852 kJ
Total = 3338 kJ
Reaction enthalpy change = 2640 - 3338 = -698 kJ
Worked examples
Q442-01 Which of the following is correct about the energy changes during bond breaking and bond formation?| bond breaking | bond formation | |
| A. | exothermic | endothermic |
| B. | exothermic | exothermic |
| C. | endothermic | exothermic |
| D. | endothermic | endothermic |
Answer
|
Bond breaking requires energy - it is endothermic. Bond formation releases energy - it is exothermic - Response C |
Q442-02 Which statement about bond enthalpies is correct?
- Bond enthalpies have positive values for strong bonds and negative values for weak bonds
- Bond enthalpy values are greater for ionic bonds than for covalent bonds
- Bond breaking is endothermic and bond making is exothermic
- The carbon-carbon bond enthalpy values are the same in ethane and ethene.
|
Bond breaking is endothermic and bond making is exothermic Response C |
Q442-03 The average bond enthalpy of the C- H bond is 412 kJ mol-1 . Which process has an enthalpy change closest to this value?
- CH4(g) → C(s) + 2H2(g)
- CH4(g) → C(g) + 2H2(g)
- CH4(g) → C(s) + 4H(g)
- CH4(g) → CH3(g) + H(g)
|
The process involving the breaking of one C- H bond corresponds to Response D |
Q442-04 What energy changes occur when chemical bonds are formed and broken?
- Energy is absorbed when bonds are formed and when they are broken
- Energy is released when bonds are formed and when they are broken
- Energy is absorbed when bonds are formed and released when they are broken
- Energy is released when bonds are formed and absorbed when they are broken
|
Energy is released when bonds are formed and absorbed when they are broken. Response D |
Q442-05 What is the value of ΔH in kJ mol-1 for the reaction below?
CH2=CH2 + H2 → CH3CH3
| Bond | H-H | C-C | C=C | C-H |
| bond energies / kJ mol-1 | 436 | 348 | 612 | 412 |
- 124
- 101
- -101
- -124
Enthalpy of reaction = bonds broken (endo) + bonds formed (exo) Enthalpy change of reaction = +2696 - 2820 = -124 kJ |
Q442-06 Some average bond enthalpies in kJ mol-1 are as follows: H - H = 436, Cl - Cl = 242, H - Cl = 431
What is the enthalpy change (in kJ) for the decomposition of hydrogen chloride?
2HCl → H2 + Cl2
- -184
- +184
- +247
- -247
Enthalpy change for the decomposition = 862 + (-678) = +184 kJ |
Q442-07 Given the following data:
- 1 C(s) + 2F2(g) → CF4(g)
 H1
= -680 kJmol-1
H1
= -680 kJmol-1 - 2 F2 (g) → 2F(g)
 H2= +158
kJmol-1
H2= +158
kJmol-1 - 3 C(s) → C(g)
 H3 = +715
kJmol-1
H3 = +715
kJmol-1
Calculate the average bond enthalpy (in kJmol-1) for the C-F bond. [4]
Answer|
The average bond enthalpy (in kJ mol-1) for the C-F bond is given by the process: C-F → C(g) + F(g) However, as C-F cannot exist on its own then we can calculate the average bond energy by dividing the energy change for the following process by four: CF4(g) → C(g) + 4F(g) To construct this equation from the equations given: CF4(g) appears in equation 1 on the RHS so we must reverse equation 1 and change the sign of the energy. CF4(g) → C(s) + 2F2(g) Now we need C(g). This appears in equation 3 on the RHS, which is required. So we can add equation 3 directly to the equation above: CF4(g) → C(s) + 2F2(g) CF4(g) + Finally, to finish constructing the required equation we need F(g). This appears in equation 2 on the RHS as 2F(g), BUT we must double the equation to get 4F(g) and then add it to the result of the equations addition above: CF4(g) → 2F2(g) + C(g)2F2(g) → 4F(g) CF4(g) + Cancelling terms gives us the required equation. The average bond enthalpy is this enthalpy change divided by 4 = +428 kJ |
Q442-08 The average bond enthalpies for O-O and O=O are 146 and 496 kJ mol-1 respectively. What is the enthalpy change in kJ for the reaction below?
H-O-O-H (g) → H-O-H(g) + ½O=O(g)
- -102
- +102
- +350
- +394
Notice the 2z terms cancel out on either side. The enthalpy change for the reaction = -102 kJ |
Q442-09 Consider the following reaction:
N2(g) + 3H2(g) → 2NH3(g) ![]() ΔH
ΔHo = ?
Bond enthalpies (in kJ mol-1) involved in the reaction are:
| N ≡ N | x | |
| H - H | y | |
| N - H | z |
Which calculation will give the value of ΔHo?
- x + 3y - 6z
- 6z - x +3y
- x - 3y + 6z
- x + 3y - 2z
Enthalpy change of reaction = x + 3y -6z kJ |
Q442-10 Enthalpy change may be calculated using bond enthalpies, some values of which ( in kJmol-1 ) are provided below:
C=C 612; C-H 412; O-H 463; C=O 743; O=O 496.
The balanced equation for the complete combustion of one mole of ethene, C2H4, in oxygen is shown below. Use this data to calculate the enthalpy of combustion of ethene:
C2H4(g) + 3O2(g) → 2CO2 + 2H2O(g)
Answer
Enthalpy change for the combustion of ethene = -1076 kJ |
| Now test yourself |