Standard level
Syllabus ref: R1.3.3Reactivity 1.3.3 - Fossil fuels include coal, crude oil and natural gas, which have different advantages and disadvantages.
- Evaluate the amount of carbon dioxide added to the atmosphere when different fuels burn.
- Understand the link between carbon dioxide levels and the greenhouse effect.
Guidance
- The tendency for incomplete combustion and energy released per unit mass should be covered.
Tools and links
- Structure 3.2 - Why do larger hydrocarbons have a greater tendency to undergo incomplete combustion?
- AHL Structure 3.2 - Why is carbon dioxide described as a greenhouse gas?
- Nature of science, Reactivity 3.2 - What are some of the environmental, economic, ethical and social implications of burning fossil fuels?

Fuels
Fuels are substances that can be used to transform an energy store into heat and/or light energy.
Types of Fuels:
- Fossil Fuels: Coal, oil, and natural gas formed from the remains of ancient plants and animals.
- Biofuels: Fuels derived from living or recently living organisms, such as ethanol and biodiesel.
- Nuclear Fuels: Uranium and plutonium used in nuclear reactors to produce energy.
- Renewable Fuels: Solar, wind, hydro, and geothermal energy sources that are replenished naturally.
Fossil Fuels
- Coal: A solid fossil fuel used mainly for electricity generation and steel production.
- Types of Coal: Lignite, bituminous, anthracite.
- Oil (Petroleum): A liquid fossil fuel used for transportation fuels like gasoline, diesel, and jet fuel.
- Refining Process: Oil is refined into different products through distillation.
- Natural Gas: A gaseous fossil fuel used for heating, electricity, and as a chemical feedstock.
- Components: Mainly methane (CH4).
Combustion
- Definition: A chemical reaction where a fuel reacts with oxygen to produce heat, light, carbon dioxide (CO2), and water (H2O).
- Combustion Equation: For methane, CH4 + 2O2 → CO2 + 2H2O + energy.
- Complete vs. Incomplete Combustion:
- Complete Combustion: Produces CO2 and H2O.
- Incomplete Combustion: Produces CO (carbon monoxide), soot (carbon particles), and less energy.
Environmental Impact
- Air Pollution: Burning fossil fuels releases pollutants like sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter.
- Greenhouse Gases: CO2 from fossil fuels contributes to global warming and climate change.
- Acid Rain: SO2 and NOx can form acids in the atmosphere, causing acid rain, which harms ecosystems.
Alternative Fuels
- Biofuels:
- Ethanol: Alcohol made from fermentation of sugars in crops like corn and sugarcane.
- Biodiesel: Made from vegetable oils or animal fats.
- Hydrogen: Can be used in fuel cells to produce electricity with water as the only by-product.
- Electricity: Generated from renewable sources like wind, solar, and hydro.
Energy Efficiency and Conservation
- Energy Efficiency: Using technology that requires less energy to perform the same function.
- Examples: LED lights, energy-efficient appliances.
- Conservation: Reducing energy use through behavior changes.
- Examples: Turning off lights, using public transportation.
Future of Fuels
- Sustainable Energy: Focus on reducing dependence on fossil fuels and increasing use of renewable energy sources.
- Technological Advancements: Development of more efficient energy storage systems, like advanced batteries.
- Policy and Regulation: Government initiatives to promote clean energy and reduce carbon emissions.
Key Concepts
- Renewable vs. Non-Renewable: Renewable fuels are replenished naturally, while non-renewable fuels are finite.
- Carbon Footprint: The total amount of greenhouse gases produced directly and indirectly by human activities.
Energy Content
The energy content of fossil fuels refers to the amount of energy that can be obtained from burning a specific amount of fuel. This energy is usually measured in terms of heat output, and the specific energy content is often given in units like joules (J), British thermal units (BTU), or calories (cal).
Specific Energy Content
The specific energy content, also known as the energy density, is a measure of the amount of energy stored in a given mass of fuel. It is usually expressed in megajoules per kilogram (MJ/kg) or BTU per pound (BTU/lb).
Coal
- Types of Coal:
- Lignite: 10-20 MJ/kg
- Bituminous: 24-35 MJ/kg
- Anthracite: 30-36 MJ/kg
- Coal has a high energy density, making it a widely used fuel for electricity generation and industrial processes.
Oil (Petroleum)
- Crude Oil: 42-47 MJ/kg
- Gasoline: 44-46 MJ/kg
- Diesel: 43-46 MJ/kg
- Oil has a high specific energy content, making it a primary fuel for transportation.
Natural Gas
- Methane (CH4): 50-55 MJ/kg
- Natural gas is highly efficient and burns cleaner than coal and oil, producing less CO2 per unit of energy.
Energy Efficiency and Comparisons
- When comparing the energy content of different fossil fuels, natural gas has the highest specific energy content, followed by oil, and then coal.
- The choice of fuel depends on factors such as availability, cost, and environmental impact.
Environmental Considerations
- While fossil fuels provide high energy content, they also contribute to environmental pollution and greenhouse gas emissions.
- Transitioning to renewable energy sources can help reduce the environmental impact and ensure sustainable energy supply.
Disadvantages of Fossil Fuels
Fossil fuels, while being a significant source of energy, have numerous disadvantages, particularly in terms of pollution and harmful by-products.
Pollution
- Air Pollution: Burning fossil fuels releases a variety of pollutants into the atmosphere.
- Sulfur Dioxide (SO2): Contributes to respiratory problems and the formation of acid rain.
- Nitrogen Oxides (NOx): Lead to smog formation and respiratory issues.
- Particulate Matter (PM): Tiny particles that can penetrate deep into the lungs, causing health issues such as asthma and heart disease.
- Volatile Organic Compounds (VOCs): Contribute to the formation of ground-level ozone and smog.
- Greenhouse Gas Emissions: Fossil fuels are a major source of greenhouse gases, which contribute to global warming and climate change.
- Carbon Dioxide (CO2): The primary greenhouse gas produced by burning fossil fuels.
- Methane (CH44): A potent greenhouse gas released during the extraction and transportation of natural gas.
- Water Pollution: Fossil fuel extraction and use can contaminate water sources.
- Oil Spills: Accidental spills during drilling or transportation can have devastating effects on marine ecosystems.
- Coal Mining Runoff: Can contaminate rivers and streams with heavy metals and other pollutants.
By-products
- Fly Ash and Bottom Ash: By-products of coal combustion that contain toxic metals and require proper disposal to prevent environmental contamination.
- Sludge: Produced in the refining of oil and the cleaning of emissions from power plants, containing hazardous substances.
- Carbon Monoxide (CO): A toxic by-product of incomplete combustion that can cause poisoning and is harmful to human health.
- Soot: Fine black particles produced from incomplete combustion, contributing to respiratory problems and environmental damage.
Environmental and Health Impact
- Fossil fuel pollution has significant health impacts, causing respiratory and cardiovascular diseases, and premature deaths.
- Environmental degradation includes acid rain, loss of biodiversity, and climate change impacts such as extreme weather events and rising sea levels.
Economic and Social Costs
- Health care costs and loss of productivity due to pollution-related illnesses.
- Cleanup and remediation costs for oil spills, coal ash ponds, and other environmental damages.
- Social impacts, including displacement of communities due to mining operations and environmental degradation.
Conclusion
While fossil fuels have been crucial for industrial development, their disadvantages in terms of pollution and harmful by-products highlight the need for cleaner, sustainable energy alternatives.
Greenhouse Effect
The greenhouse effect is a natural process that warms the Earth's surface. It occurs when certain gases in the Earth's atmosphere trap heat from the sun, preventing it from escaping back into space.
Greenhouse Gases
- Carbon Dioxide (CO2): Released from burning fossil fuels, deforestation, and various industrial processes.
- Methane (CH4): Emitted during the production and transport of coal, oil, and natural gas, as well as from livestock and other agricultural practices.
- Nitrous Oxide (N2O): Released from agricultural and industrial activities, as well as during combustion of fossil fuels and solid waste.
- Chlorofluorocarbons (CFCs): Synthetic compounds used in air conditioning, refrigeration, and aerosol propellants that also contribute to ozone layer depletion.
- Water Vapor (H2O): The most abundant greenhouse gas, but its concentration in the atmosphere is mainly controlled by natural processes.
How the Greenhouse Effect Works
- Sunlight enters the Earth's atmosphere and reaches the surface, where it is absorbed and then re-radiated as heat (infrared radiation).
- Greenhouse gases absorb and re-emit this heat, trapping it in the atmosphere and warming the planet.
- This natural process keeps the Earth's temperature at a level that can support life.
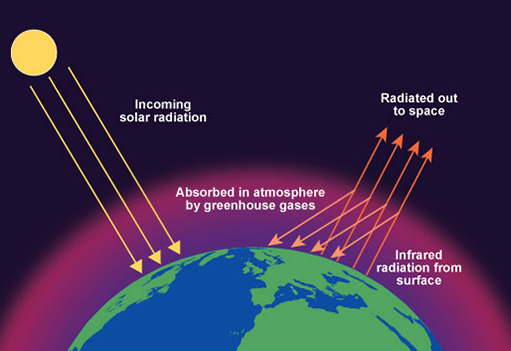
Enhanced Greenhouse Effect
Human activities, especially the burning of fossil fuels and deforestation, have increased the concentrations of greenhouse gases in the atmosphere, leading to an enhanced greenhouse effect.
- Global Warming: The increase in Earth's average surface temperature due to rising levels of greenhouse gases.
- Climate Change: Long-term changes in temperature, precipitation, and other atmospheric conditions resulting from the enhanced greenhouse effect.
Consequences of the Enhanced Greenhouse Effect
- Extreme Weather Events: Increased frequency and intensity of hurricanes, heatwaves, droughts, and heavy rainfall.
- Rising Sea Levels: Melting polar ice caps and glaciers, along with the thermal expansion of seawater, contribute to rising sea levels, threatening coastal communities.
- Ocean Acidification: Increased CO2 absorption by the oceans leads to acidification, affecting marine life and ecosystems.
- Loss of Biodiversity: Changes in climate and habitat loss threaten species survival and disrupt ecosystems.
- Human Health Impacts: Increased heat-related illnesses, spread of vector-borne diseases, and food and water scarcity.
Mitigation and Adaptation
- Reducing Emissions: Transitioning to renewable energy sources, improving energy efficiency, and adopting sustainable agricultural practices.
- Carbon Sequestration: Capturing and storing CO2 from the atmosphere through reforestation, soil management, and technological solutions like carbon capture and storage (CCS).
- Adaptation Strategies: Building resilient infrastructure, developing early warning systems, and implementing water conservation measures to cope with the impacts of climate change.
Conclusion
The greenhouse effect is essential for life on Earth, but human activities have intensified this natural process, leading to global warming and climate change. Addressing these challenges requires concerted efforts to reduce greenhouse gas emissions and adapt to the changing climate.
Worked examples
Q461-01 Which of the changes below occurs with the greatest increase in entropy?- Na2O(s) + H2O(l) → 2Na+(aq) + 2OH-(aq)
- NH3(g) + HCl(g) → NH4Cl(s)
- H2(g) + I2(g) → 2HI(g)
- C(s) + CO2(g) → 2CO(g)
|
Entropy can be considered the degree of disorder of a chemical system. It is increased by the number of particles and their temperature. In this case it is important to examine the number of moles of free particles on both sides of the equation. It may be seen that in equation D there are more moles of gas (maximum entropy) on the right hand side than on the left hand side. Thus the entropy increases from left to right. correct response Although there are more free ions in A this is not as important in entropy terms as an increase in the number of moles of gas. In equation B there is a large decrease in entropy (two gases make a solid) and in equation C the number of moles of gas on both sides is equal. |
Q461-02 In which of the following reactions is the entropy change ( S) closest to zero
- SO2(g) + ½O2(g) → SO3(g)
- Br2(l) → Br2(g)
- H2(g) + I2(g) → 2HI(g)
- 3Ca(s) + N2 → Ca3N2(s)
|
Entropy can be considered the degree of disorder of a chemical system. It is increased by the number of particles and their temperature. In this case it is important to examine the number of moles of free particles, i.e. gas, on both sides of the equation. Equation A the moles of gas decreases from reactants to products, ΔS is negative. Equation B the moles of gas increases from 0 to 1, ΔS is positive. Equation C the moles of gas stays the same from reactants to products, ΔS = 0. correct response Equation D the moles of gas decreases from 1 to 0, ΔS is negative. |
Q461-03 Estimate, without doing a calculation, the magnitude of the entropy change for the following reaction.
Fe2O3(s) + 2Al(s)  2Fe(s) + Al2O3(s)
2Fe(s) + Al2O3(s) |
|
Examination of the equation reveals that the compounds on both sides of the equation are in the solid state. As solids have very low entropy it is safe to estimate that the entropy difference between reactants and products is negligible. Hence ΔS = 0. |
Q461-04 Consider the following reaction:
| N2(g) + 3H2(g) → 2NH3(g) |
The absolute entropy values, S, at 300K for N2(g), H2(g)
and NH3(g) are 193, 131 and 192 JK-1 mol-1
respectively. Calculate ΔSo
for the reaction and explain the sign of So.
|
On the left hand side there is one mole of nitrogen and three moles of hydrogen. Their entropy = 193 + (3 x 131) = 586 JK-1 On the right hand side there are two moles of ammonia. Entropy = (2 x 192) = 384 JK-1 The entropy change, ΔS The negative sign indicates that the entropy has decreased from reactants to products. |
Q461-05 Which reaction has the greatest positive entropy change?
- CH4(g) + 1½O2(g) → CO(g) + 2H2O(g)
- CH4(g) + 1½O2(g) → CO(g) + 2H2O(l)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
|
A positive entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 2½ moles gas → 3 moles of gas. An increase by ½ mole gas correct response reaction 2 2½ moles gas → 1 mole of gas. A decrease of 1½ mole gas reaction 3 3 moles gas → 3 moles of gas. No change in moles reaction 4 3 moles gas → 1 mole of gas. A decrease of 2 moles of gas |
Q461-06 Which reaction occurs with the largest increase in entropy?
- Pb(NO3)2(s) + 2KI(s) → PbI2(s) + 2KNO3(s)
- CaCO3(s) → CaO(s) + CO2(g)
- 3H2(g) + N2(g) → 2NH3(g)
- H2(g) + I2(g) → 2HI(g)
|
An increase in entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 0 moles gas → 0 moles of gas. No change in moles of gas reaction 2 0 moles gas → 1 mole of gas. A increase of 1 mole of gas correct response reaction 3 4 moles gas → 2 moles of gas. A decrease of 2 moles of gas reaction 4 2 moles gas → 2 mole of gas. No change in moles of gas |
Q461-07 Some chlorine gas is placed in a flask of fixed volume at room temperature. What change will cause a decrease in entropy?
- Adding a small amount of hydrogen
- Adding a small amount of chlorine
- Cooling the flask
- Exposing the flask to sunlight
|
Anything that increases the disorder, such as mixing two gases, or increasing the temperature, increases the entropy. The reverse is also tru. Hence decreasing the temperature decreases the entropy, e.g. Cooling the flask |
Q461-08 Which reaction has the largest positive value of ΔS
- CO2(g) + 3H2(g) → CH3OH(g) + H2O(g)
- 2Al(s) + 3S(s) → Al2S3(s)
- CH4(g) + H2O(g) → 3H2(g) + CO(g)
- 2S(s) + 3O2(g) → 2SO3(g)
|
An increase in entropy change means that the products have more entropy than the reactants. Gases have the largest entropy values, therefore we are looking for the reaction that produces the greatest positive change in moles of gas. reaction 1 4 moles gas →
2 moles of gas. A decrease of 2 moles of gas, ΔS reaction 2 0 moles gas →
0 mole of gas. No change in moles of gas, ΔS reaction 3 2 moles gas →
4 moles of gas. An increase of 2 moles of gas, ΔS reaction 4 3 moles gas →
2 mole of gas. A decrease of 1 mole of gas, ΔS |
Q461-09 Which equation represents a change with a negative value for ΔS?
- 2H2(g) + O2(g) → 2H2O(g)
- H2O(s) → H2O(g)
- H2(g) + Cl2(g) → 2HCl(g)
- 2NH3(g) → N2(g) + 3H2(g)
|
A negative value for ΔS means that the products have less entropy than the reactants. There are fewer moles of gas in the products than in the reactants. reaction 1 3 moles gas → 2 moles of gas. A decrease of 1 moles of gas, ΔS = negative correct response reaction 2 0 moles gas → 1 mole of gas. An increase by 1 mole of gas, ΔS = positive reaction 3 2 moles gas → 2 moles of gas. No change in moles of gas , ΔS = 0 (approx) reaction 4 2 moles gas → 4 mole of gas. An increase by 2 moles of gas, ΔS = positive |
Q461-10 Which change does not lead to an increase in entropy?
- Mixing nitrogen and oxygen gases at room temperature
- Cooling steam so that it condenses to water
- Heating hexane to its boiling point
- Dissolving sugar in water
|
Entropy is increased by:
From the choices given, only cooling steam reduces the entropy of the system |