Standard level
A chemical change may be defined as: "A process in which a new substance is formed"
Syllabus ref: R2.1.3Reactivity 2.1.3 - The limiting reactant determines the theoretical yield.
- Identify the limiting and excess reactants from given data.
Guidance
- Distinguish between the theoretical yield and the experimental yield.
Tools and links
- Tool 1, Inquiry 1, 2, 3 - What errors may cause the experimental yield to be i) higher and ii) lower than the theoretical yield?
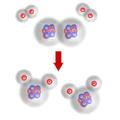
Excess reagent
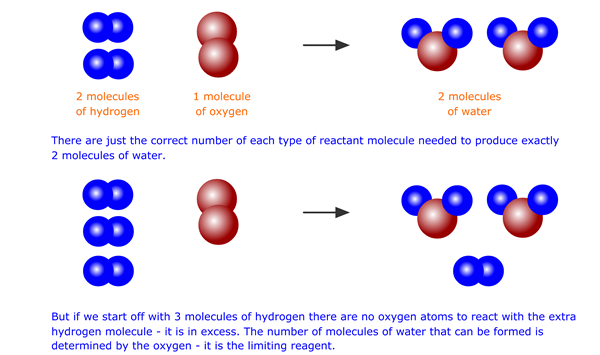
When there is more of one of the reactants present than the required amount, the extra will not have anything to react with. This should be apparent bearing in mind the particulate nature of matter. If two molecules of hydrogen react with one molecule of oxygen to make two molecules of water, then any 'extra' molecules of hydrogen (or oxygen for that matter) will be unable to react.
Three hydrogen molecules trying to react with one oxygen molecule
Here you can see that the extra hydrogen molecule (at the lower right of the diagram) remains unreacted. We call this the excess reagent.
An extra amount of chemical over and above that which is needed for complete reaction is called the excess. A reagent in excess (i.e. one of which there is more than the amount needed) cannot completely react. Some of it can react, but the rest simply remains unreacted after the reaction has finished.
Calculating the excess
To find the excess reagent, the first stage is to calculate the number of moles of each reagent in the reaction. Then the stoichiometry of the equation shows the relative number of moles reacting in an ideal situation. The excess is found by substituting the number of moles of the first reagent (reacting chemical) in the given situation and seeing how many moles of the second reagent is required for complete reaction.
If there is more than enough of the second reagent then it is in excess. If there is not enough of the second reagent then the first reagent is in excess.
Example: 64 grams of sulfur react with 64 grams of iron
Fe + S → FeS
1 mole of iron reacts completely with 1 mole of sulfur.
RAM Fe = 56, RAM S=32.
From the quantities given we have 64/32 moles of sulfur and 64/56 moles of iron
i.e. 2 moles of sulfur and 1.143 moles of iron
But as they always react in a 1 : 1 mole ratio then 2 moles of sulfur would need 2 moles of iron. Clearly there is not enough iron to react with all the sulfur. Therefore some of the sulfur must remain unreacted once all of the iron is used up.
The sulfur is said to be in excess.
Only 1.143 moles of the sulfur can react with 1.143 moles of iron.
This means that 2 - 1.143 moles of sulfur is left unreacted = 0.857 moles
Limiting reagent
In the above example it can be seen that the component that determines the amount of sulfur that can react is the iron. All of the iron manages to react and it is this that determines the quantity of products formed. The iron is said to LIMIT the reaction. It is called the limiting reagent.
To determine the limiting reagent (and to find out which of the reactants is in excess) the stoichiometry of the reaction must be considered.
Procedure
- Firstly find the relative number of moles of each component in the balanced equation.
- Then convert the data given in the question under study into moles.
- Now by inspection see which one of the components will completely react and which one will be in excess.
Example: 25cm3 of 0.2 M sodium hydroxide solution is mixed with 80 cm3 of 0.05 M sulfuric acid.
2NaOH + H2SO4 → Na2SO4 + 2H2O
From the equation 2 moles of sodium hydroxide react completely with 1 mole of sulfuric acid
From the data given:
25cm3 of 0.2 M sodium hydroxide = 0.025 x 0.2 moles = 0.005 moles
80 cm3 of 0.05 M sulfuric acid = 0.08 x 0.05 = 0.004 moles
From the equation mole ratios, 0.005 moles of sodium hydroxide would react completely with 0.005/2 = 0.0025 moles of sulfuric acid. But there are 0.004 moles of sulfuric acid (i.e. more than enough). Some of the sulfuric acid will remain unreacted - it is in EXCESS.
The LIMITING reagent is the sodium hydroxide as it is this alone that determines the amount of product formed
Worked examples - limiting reagent
Find the limiting reagent in each of the following questions
Q361-01 15 g of calcium metal burns in 11.35 dm3 of oxygen (measured at STP, 1 mole of gas occupies 22.7 dm3)
Answer|
Equation for the reaction: 2Ca + O2 → 2CaO mole ratio from the equation: 2 moles of calcium reacts completely with 1 mole of oxygen gas. Data given [RAM: Ca = 40] Moles of calcium = 15/40 = 0.375 moles Moles of oxygen = 11.35/22.7 = 0.5 moles From the equation: 0.375 moles of calcium would require 0.375/2 moles of oxygen = 0.1875 moles There is more than enough oxygen so it is in EXCESS The LIMITING reactant is the calcium |
Q361-02 12.35 g of copper carbonate dissolves in 500 cm3 of 0.1M HCl
Answer|
Equation for the reaction: CuCO3 + 2HCl → CuCl2 + H2O + CO2 mole ratio from the equation: 1 moles of copper carbonate reacts completely with 2 moles of HCl. Data given [Mr : CuCO3 = 63.5 + 12 + (3 x 16) = 123.5] Moles of CuCO3 = 12.35/123.5 = 0.1 moles Moles of HCl = 0.5 x 0.1 = 0.05 moles From the equation: 0.1 moles of CuCO3 would require 0.2 moles of HCl There is not enough HCl so it is the LIMITING reactant. The copper carbonate is in excess. |
Q361-03 1.2 g of magnesium is dissolved in 50 cm3 of 2M sulfuric acid.
Answer|
Equation for the reaction: Mg + H2SO4 → MgSO4 + H2 Ar of magnesium = 24, therefore 1.2 g represents 1.2/24 moles = 0.05 moles From the equation, 1 mole of magnesium requires 1 mole of sulfuric acid Therefore 0.1 moles magnesium requires 0.1 moles of sulfuric acid. 50 cm3 of 2M sulfuric acid is equivalent to 0.05 x 2 = 0.1 moles of sulfuric acid. There is more than enough sulfuric acid - it is in EXCESS The limiting reagent is the magnesium |
Q361-04 7.95 g of copper(II) oxide is dissolved in 50 cm3 of 2M hydrochloric acid.
Answer|
Equation for the reaction: CuO + 2HCl → CuCl2 + H2O Mr of copper(II) oxide = 63.5 + 16 = 79.5 7.95 g represents 7.95/79.5 moles = 0.1 moles From the equation, 1 mole of copper oxide requires 2 moles of hydrochloric acid Therefore 0.1 moles copper oxide requires 0.2 moles of hydrochloric acid. 50 cm3 of 2M hydrochloric acid is equivalent to 0.05 x 2 = 0.1 moles of hydrochloric acid. There is not enough hydrochloric acid to react with all the copper(II) oxide - it is the LIMITING REAGENT The copper oxide is in EXCESS. |
Q361-05 1.27 g of iodine reacts with 2.7g aluminium metal [I=127, Al=27]
Answer|
Equation for the reaction: 2Al + 3I2 → 2AlI3 2 moles of aluminium require 3 moles of iodine. 2.7 g aluminium is equal to 2.7/27 moles aluminium = 0.1 moles this would require 0.1 x 3/2 = 0.15 moles of iodine 1.27 g iodine = 1.27/254 moles = 0.005 moles iodine. There is not enough iodine to react with all of the aluminium. The iodine is the LIMITING REAGENT. The aluminium is in EXCESS. |
Q361-06 2.27 dm3 of chlorine gas is absorbed by 100cm3 of 2M sodium hydroxide (measured at STP, 1 mole of gas occupies 22.7 dm3)
Answer|
Equation for the reaction NaOH + Cl2 → NaOCl + HCl one mole of chlorine requires 1 mole of sodium hydroxide Moles of chlorine = 2.27/22.7 = 0.1 moles This would require 0.1 moles NaOH Moles of NaOH = 0.1 x 2 = 0.2 moles There is more than enough NaOH - it is in EXCESS. The chlorine is the LIMITING REAGENT. |
Q361-07 11.35 dm3 of ammonia gas is absorbed by 1dm3 of 2M sulfuric acid (measured at STP, 1 mole of gas occupies 22.7 dm3)
Answer|
Equation for the reaction 2NH3 + H2SO4 → (NH4)2SO4 2 moles of ammonia require 1 mole of sulfuric acid Moles of ammonia = 11.35/22.7 = 0.5 moles This would require 0.5/2 moles of sulfuric acid = 0.25 moles Moles of sulfuric acid = 1 x 2 = 2 moles There is more than enough sulfuric acid - it is in EXCESS. The ammonia is the LIMITING REAGENT. |
Q361-08 45.4 dm3 of carbon dioxide gas is absorbed by 1dm3 of 2M sodium hydroxide (measured at STP, 1 mole of gas occupies 22.7 dm3)
Answer|
Equation for the reaction CO2 + 2NaOH → Na2CO3 + H2O 1 mole of carbon dioxide requires 2 moles of sodium hydroxide Moles of carbon dioxide = 45.4/22.7 = 2 moles This would require 2 x 2 moles of sodium hydroxide = 4 moles Moles of sodium hydroxide = 1 x 2 = 2 moles There is not enough sodium hydroxide - it is the LIMITING REAGENT The carbon dioxide is in EXCESS.. |
Q361-09 1.27 g of iodine [I=127] reacts with 50cm3 of 0.5M sodium thiosulfate according to the equation:
I2 + 2Na2S2O3 → Na2S4O6 + 2NaI
Answer|
Mr of I2 = 2 x 127 = 254 Moles of iodine present = 1.27/254 = 0.005 moles From the equation 1 mole of iodine requires 2 moles of sodium thiosulfate Therefore 0.005 moles of iodine would require 0.005 x 2 = 0.01 moles of sodium thiosulfate Moles of sodium thiosulfate = 0.05 x 0.5 moles = 0.025 moles This is more than enough sodium thiosulfate to react with all of the iodine. The sodium thiosulfate is in EXCESS. The iodine is the LIMITING REAGENT |
Q361-10 In a thermite reaction 5.4 g of aluminium reduces 12.0 g of iron(III) oxide. Calculate the limiting reagent and the mass of iron produced. [Ar: Fe=56, Al=27, O=16]
Answer|
Equation for the reaction: 2Al + Fe2O3 → Al2O3 + 2Fe 2 moles of aluminium require 1 mole of iron(III) oxide. From the data given Moles of aluminium = 5.4/27 = 0.2 moles This would require 0.2/2 = 0.1 moles of iron(III) oxide Mr:iron(III) oxide = (56 x 2) + (16 x 3) = 160 12.0 g of iron(III) oxide = 12.0/160 = 0.075 moles This is not enough to react with all of the aluminium therefore the iron(III) oxide is the LIMITING REAGENT. The limiting reagent determines the amoiunt of final product. There is 0.075 moles of iron(III) oxide and from the equation we see that this will produce twice as many moles of iron. Therefore moles of iron produced = 2 x 0.075 = 0.15 moles. Mass of iron produced = moles x 56 Mass of iron produced = 0.15 x 56 = 8.4 g |
Now test yourself