Higher-level only
Collision theory requires that particles collide. However, not all collisions lead to successful reaction even if they have enough energy to overcome the activation energy barrier. The Arrhenius factor takes this into account.
Syllabus ref: R2.2.13Reactivity 2.2.13 - The Arrhenius factor, A, takes into account the frequency of collisions with proper orientations. (HL)
- Determine the activation energy and the Arrhenius factor from experimental data.
Guidance
Tools and links
Arrhenius Factor
The Arrhenius factor, also known as the pre-exponential factor (A), is a crucial component in the Arrhenius equation. It helps describe the rate of a chemical reaction.
Arrhenius Equation
The Arrhenius equation is given by:
- k: Rate constant of the reaction
- A: Pre-exponential factor or Arrhenius factor
- Ea: Activation energy
- R: Universal gas constant (8.314 J/mol·K)
- T: Temperature in Kelvin
Understanding the Arrhenius Factor (A)
The Arrhenius factor is a measure of the frequency of collisions and the orientation of reacting molecules. It represents the number of times reactants collide with the proper orientation to form products.
- High value of A indicates a large number of effective collisions.
- Depends on factors like the nature of the reactants and the complexity of the reaction mechanism.
Factors Influencing the Arrhenius Factor
- Nature of reactants
- Simple molecules generally have higher A values.
- Complex molecules may have lower A values due to steric factors.
- Reaction Mechanism
- Elementary reactions usually have higher A values.
- Multi-step reactions might have lower A values.
Significance of the Arrhenius Factor
The Arrhenius factor is essential for understanding the kinetics of a reaction:
- Helps in calculating the rate constant (k) of a reaction.
- Provides insight into the reaction mechanism.
- Assists in predicting how reaction rates change with temperature.
Practical Applications
- In industrial chemistry for optimizing reaction conditions.
- In pharmaceuticals for understanding drug stability and reaction rates.
- In environmental science for studying pollutant degradation rates.
Worked examples
Q642-01 For irreversible reactions, the rate will be affected by changes in all of these factors except- temperature.
- concentration of reactants.
- presence of a catalyst.
- concentration of products.
|
The concentration of products cannot affect the rate of the forward reaction if it is irreversible. In reality there are no irreversible reactions, rather they are irreversible under the chosen conditions. |
Q642-02 The rate of the reaction: 2NO + Cl2 → 2NOCl is given by the rate equation, rate = k[NO]2[Cl2]. The value of the rate constant can be increased by:
- increasing the concentration of the NO.
- increasing the concentration of the Cl1.
- increasing the temperature.
- doing all of these.
|
The rate constant is independent of concentrations, but does depend on the temperature. |
Q642-03 A small increase in temperature often causes a large increase in the rate of a chemical reaction. This effect is best attributed to
- a decrease in the activation energy of the reaction
- more frequent collisions at the higher temperature
- the occurrence of more collisions with the needed energy
- different reaction pathways at the higher temperature
|
Activation energy cannot be affected except by providing an alternative pathway (mechanism) using a catalyst There are more collisions as the temperature rises, but this is not as important as the success of the collisions. The increase in temperature means that more particles will have the required energy to make their collisions successful There is no reason to suppose that higher temperatures lead to different mechanisms |
Q642-04 Zinc metal reacts with excess HCl according to the equation:
Zn(s) + 2H+(aq) + 2Cl-(aq) → Zn2+(aq) + 2Cl-(aq) + H2(g)
Which of the following change will increase the rate of evolution of H2?
- I. using zinc dust in place of chunks
- II. using 2 M HCl in place of 1 M HCl
- III. using 200 mL of 1 M HCl in place of 100 mL
- I only
- I and II only
- II and III only
- I, II, and III
|
Using dust in place of lumps increases the available surface area for reaction. Increasing the concentration increases the rate as seen by the rate expression (this would not apply for 0th order components), however increasing the volume has no effect on the rate. I and II only |
Q642-05 Ingold was awarded a Nobel Prize for his investigations into the kinetics of the hydrolysis of bromoalkanes in alkaline aqueous ethanol.
RBr + OH- → ROH + Br-
He obtained the following rate constants for the hydrolysis of bromoalkanes.
| - | CH3Br | C2H5Br | CH3CHBrCH3 | (CH3)3CBr |
| First order | - | - | 1.4 x 104 | 1.0 x 102 |
| Second order | 1.1 x 102 | 7.1 x 103 | 4.7 x 105 | - |
Deduce the initial rate of hydrolysis of bromoethane, if 50 cm3 of a 0.1 mol dm-3 ethanolic solution of bromoethane is completely mixed with 50 cm3 of alkaline aqueous ethanol, which has a concentration of 0.05 mol dm-3 with respect to hydroxide ions.
Answer|
Bromoethane, C2H5Br, in the table undergoes hydrolysis via a second order process. Therefore the rate expression is: Rate = k[C2H5Br]1[OH-]1 Moles of bromoethane in the mixture = 0.1 x 0.05 = 0.005 moles This is in 50 + 50 = 100 cm3 therefore the initial concentration of bromoethane = 0.005/0.1 = 0.05 mol dm-3 Concentration of hydroxide ions initially = 0.05 x 0.05/0.1 = 0.025 mol dm-3 Substituting the values in the rate equation: Rate = k[C2H5Br]1[OH-]1 Rate = 7.1 x 103 x 0.05 x 0.025 Therefore initial rate = 8.875 mol dm-3 s-1 |
Q642-06 A small increase in temperature often produces a large increases in the rate of a chemical reaction because it:
- decreases the activation energy of the reaction.
- increases the effectiveness of the collisions between the reactant molecules
- decreases the number of collisions per second between the reactant molecules
- decreases the volume of the solution, altering the concentrations of the reactants.
|
Increasing the temperature gives more energy to the particles and increases the effectiveness of the collisions. |
Q642-07 The reaction between nitrogen and oxygen in the atmosphere under normal conditions is extremely slow. Which statement best explains this?
- The concentration of oxygen is much lower than that of nitrogen
- The molar mass of nitrogen is less than that of oxygen
- The frequency of collisions between nitrogen and oxygen molecules is lower that that between nitrogen molecules themselves
- Very few nitrogen and oxygen molecules have sufficient energy to react
|
Nitrogen is an unreactive gas due to its strong triple bond that must be broken for reaction to occur. Very few nitrogen and oxygen molecules have sufficient energy to react |
Q642-08 The rate of the reaction of a strip of magnesium ribbon and 50cm3 of 1.0 mol dm-3 HCl is determined at 25ºC. In which case would both new conditions contribute to an increase in rate?
- Mg powder and 100cm3 of 1 mol dm-3 HCl
- Mg powder and 100cm3 of 0.8 mol dm-3 HCl
- 100cm3 of 1 mol dm-3 HCl at 30ºC
- 50cm3 of 1.2 mol dm-3 HCl at 30ºC
|
decreasing particle size to powder increases the rate. Increasing the concentration of the acid increases the rate. Increasing the temperature increases the rate. The only choice that has two rate increasing factors is D, 50cm3 of 1.2 mol dm-3 HCl at 30ºC. |
Q642-09 Doubling which of the following will double the rate of a first order reaction?
- Concentration of the reactant
- Size of the solid particles
- Volume of the solution in which the reaction is carried out
- Activation energy
|
First order reactions have the form rate = k[A], therefore doubling the concentration of the reactant doubles the rate. |
Q642-10 The curve in the diagram is obtained for the reaction of an excess of CaCO3 with hydrochloric acid. How and why does the rate of reaction change with time?
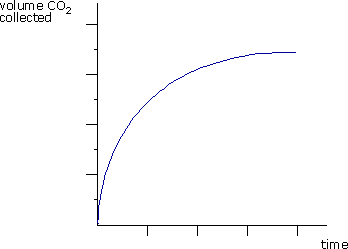
| Rate of reaction | Reason | |
| A. | decreases | HCl becomes more dilute |
| B. | decreases | The pieces of CaCO3 become smaller |
| C. | increases | The temperature increases |
| D. | increases | The CO2 produced acts as a catalyst |
|
The rate decreases over time as the reactants get used up. In this case the HCl is becoming more dilute. |