Standard level
A catalyst is a substance that affects the rate of a chemical reaction and can be recovered unchanged chemically at the end of the reaction.
Syllabus ref: R2.2.5Reactivity 2.2.5 - Catalysts increase the rate of reaction by providing an alternative reaction pathway with lower Ea.
- Sketch and explain energy profiles with and without catalysts for endothermic and exothermic reactions.
- Construct Maxwell–Boltzmann energy distribution curves to explain the effect of different values for Ea on the probability of successful collisions.
Guidance
- Biological catalysts are called enzymes.
- The different mechanisms of homogeneous and heterogeneous catalysts will not be assessed.
Tools and links
- Reactivity 2.3 - What is the relative effect of a catalyst on the rate of the forward and backward reactions?
- AHL Structure 3.1 - What are the features of transition elements that make them useful as catalysts?
Catalysis
Catalysts increase the rate of chemical reactions.
They may be in the same phase as the reactants (homogeneous) or in a different phase (heterogeneous).
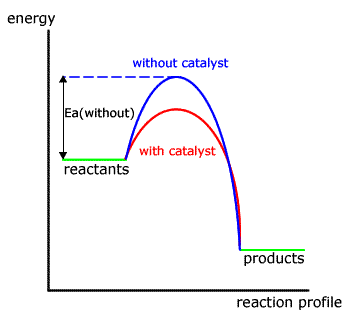
Both forms of catalyst act in a similar way, reducing the activation energy needed for a particular reaction by offering an alternative mechanism.
In the energy profile for a non-catalysed reaction the path taken is shown by the blue hump.
When a catalyst is added the different mechanism has a lower activation energy, hence there are more particles with sufficient energy to react and the reaction is faster.
In neither case is the energy of the reactants or products affected.
'd' block elements make good catalysts due to their multiple oxidation states (hence their ability to react with different species and produce a path of lower activation energy, and so allow the reaction to proceed at a faster rate).
Another possible reason for their catalytic activity could be their available empty '4d' orbitals, which allow reacting molecules to co-ordinate to the surface of the transition metal. This, in turn, weakens the bonding within the molecule encouraging reaction.
|
Examples
|
Decomposition of hydrogen peroxide
Hydrogen peroxide, H2O2, is an unstable, colourless liquid that slowly decomposes into water and oxygen in the light. For this reason it is kept in darkened bottles. The decomposition is very slow at rooom temperature.
Manganese(IV) oxide, MnO2, is an insoluble black/brown powder. When it is added to hydrogen peroxide solution, the hydrogen peroxide decomposes rapidly and the manganese dioxide catalyst can be filtered off unchanged.
This is an example of heterogeneous catalysis.
2H2O2 → 2H2O + O2
The reaction can be followed by collecting the oxygen gas evolved.
Hydrogenation of alkenes
Alkenes contain double bonds between carbon atoms. Hydrogen can be added to these bonds using hydrogen gas at 150ºC in the presence of a nickel (or platinum) catalyst.
C2H4 + H2 → C2H6
This reaction, called hydrogenation, is useful in many processes, including the magarine industry. Margarine is made from vegetable oils. Vegetable oils consist of long, (C15-20) unsaturated alkyl chains linked to a propyl chain by ester linkages.
The melting temperature of the oils is increased by hydrogenation of the double bonds in the long alkyl chain. This allows the molecules to align more efficiently, increasing their bonding forces and consequently their melting temperature.
The Haber process
Developed by Fritz Haber in the early 20th century, the Haber process is the industrial manufacture of ammonia gas.
The process involves the reaction between nitrogen and hydrogen gases under pressure at moderate temperatures to produce ammonia. However, the reaction is an equilibrium and even under the most favourable conditions, less than 20% of ammonia gas is present.
Haber's adaptation to a well-known reaction, was to recycle the gaseous equilibrium mixture after rapid cooling to remove the ammonia.
The catalyst of choice in the reaction chamber is iron metal.
N2(g) + 3H2(g) → 2NH3(g)![]() ΔH
= -92 kJ
ΔH
= -92 kJ
The Haber process offers a means of obtaining soluble nitrogen compounds from the inert nitrogen of the air. Nitrogen compounds are of great importance in the fertilizers, drugs, explosives and many other industries.
Prior to the Haber process the majority of the worlds nitrates were obtained from 'guana', fecal deposits from sea birds, mined in great quantities on islands in the Pacific ocean. The Haber process removed this necessity.
Nowadays virtually all of the worlds supply of nitrogen compounds comes from the Haber process.
The Contact process
The contact process uses the raw materials of sulfur and air to manufacture sulfuric acid. Sulfuric acid is possibly the most important chemical in the world in terms of its incredible versatility in the manufacture of many other products.
Sulfuric acid behaves as a catalyst, an oxidising agent, a sulfonating agent, a dehydrating agent and as an acid in many diverse processes.
The contact process relies on the reaction between sulfur(IV) oxide (sulfur dioxide) and oxygen in the presence of a catalyst at 450ºC to produce sulfur trioxide.
The sulfur trixioxide is then reacted with water under special conditions to obtain sulfuric acid.
Stage 1: Sulfur from the petrochemicals industry, or volcanic mining, is burnt in air to produce sulfur dioxide
S + O2 → SO2
Stage 2: The sulfur dioxide is passed with more air over a hot catalyst of vanadium(V) oxide. This catalyses the formation of sulfur trioxide (sulfur(VI) oxide)
SO2 + O2 ⇌ SO3
(although the reaction is an equilibrium, it effectively goes 100% to the right hand side under the conditions chosen)
Stage 3: The sulfur trioxide is cooled and then dissolved in 98% sulfuric acid: 2% water. This makes the acid 100%, which can then have more water added to make 98% acid again (now with a larger volume).
The reason why water is mixed with sulfur trioxide in this way is to avoid the fine mist of sulfuric acid that could form during the extremely exothermic reaction between sulfur trioxide and water.
SO3 + H2O → H2SO4
The contact process took over from the Lead Chamber process, which was less efficient and much more expensive.
Sulfuric acid is an essential chemical for any county that harbours aspirations for industry.
| Industry | product | sulfuric acid |
| Detergent | alkyl hydrogen sulfates | sulfonating agent |
| Perfumes | esters | catalyst |
| Explosives | nitro compounds | strong acid/catalyst |
| Pharmaceuticals | many | various |
Summary questions
Q546-01 Transition metals can be distinguished from main group metals by the fact that:- transition metals compounds have a greater tendency to form coloured solutions.
- main group metals only have +1 or +2 oxidation states.
- main group metals have higher relative atomic masses than transition metals.
- most main group metals are silvery.
|
Main group metals (groups 1, 2 and 3) usually show oxidation states corresponding to the group valency, which may be 1, 2 or 3. Transition metals also have ions with +1 and +2 oxidation states. However, transition metal complexes are usually coloured whereas main group compounds are almost never coloured. Correct response = B |
Q546-02 Which of the following salts form coloured solutions when dissolved in water?
- I. ScCl3
- II. FeCl3
- III. NiCl2
- IV. ZnCl2
- I and II only
- II and III only
- III and IV only
- I, I, III and IV
|
Scandium only forms a 3+ ion which has an empty 3d sub-shell, hence no d-d transitions are possible. Zinc 2+ has a completely full set of 3d orbitals ond once again no d-d transitions are possible. Correct response = II and III only |
Q546-03 Which 'd' block ion is not coloured?
- Ni2+
- Fe2+
- Sc3+
- Cr3+
|
Scandium only forms a 3+ ion which has an empty 3d sub-shell, hence no d-d transitions are possible. Therefore Sc3+ is not coloured |
Q546-04 Which of the following best explains the colour of a transition metal compex ion in solution?
- The transition metal ions transfer electrons to the molecules in water absorbing energy
- The 3d orbitals have different energies and transitions between them are possible absorbing energy.
- Electrons are lost from the transtion metal ions absorbing energy
- The ligands transfer electrons to the transition metal ion and loses energy.
|
Colour is caused by transitions between partially occupied non-degenerate 'd' orbitals absorbing specific wavelengths of light. Therefore correct response = "the 3d orbitals have different energies and transitions between them are possible absorbing energy" |
Q546-05 Which complex ion is colourless?
- [Cr(H2O)6]3+
- [Fe(CN)6]4-
- [Cu(NH3)4]2+
- [Zn(H2O)4]2+
|
Colour is caused by transitions between partially occupied non-degenerate 'd' orbitals absorbing specific wavelengths of light. Zinc 2+ has a completely full set of 3d orbitals and consequently d-d transitions are not possible. Correct response = [Zn(H2O)4]2+ |
Q546-06 Which of the following could not act as a ligand in a complex of a d-block element?
- Cl-
- NCl3
- PCl3
- PCl5
|
Ligands must have a lone pair of electrons to be able to bond to the transition metal ion. Of the choices given only PCl5 does not have an available lone pair of electrons. |
Q546-07 In the equation below the cyanide ions act as which of the following?
[Fe(H2O)6]3+(aq) + 6CN-(aq) → [Fe(CN)6]3-(aq) + 6H2O(l)
- Brønsted bases
- Lewis acids
- Ligands
- Reducing agents
|
The cyanide ion is replacing water molecules. It is a strong ligand. |
Q546-08 Which of the following is an essential feature of a ligand?
- A negative charge
- An odd number of electrons
- The presence of two of more atoms
- The presence of a non-bonding pair of electrons
|
Ligands must have a lone pair (non-bonding pair) of electrons to be able to coordinate to the transition metal ion. |
Q546-09 Which of the following particles can act as ligands in complex ion formation?
- I. Cl-
- II. NH3
- III. H2O
- I and II only
- I and III only
- II and III only
- I, II and III
|
Ligands must have a lone pair (non-bonding pair) of electrons to be able to coordinate to the transition metal ion. Of the choices given all three have a non-bonding pair available. Correct response = I, II and III |
Q546-10 Which of the following species involves the transition metal in one of its common oxidation states?
- I. Cr2O72-
- II. MnO43-
- III. FeCl3
- I and II only
- I and III only
- II and III only
- I, II and III
|
The only one with an unusual oxidation state is the MnO43- ion, which has manganese in the Mn(V) oxidation state. Correct response = I and III only, Cr(VI) and Fe(III) |