Standard level
The 'position' of equilibrium is a concept that describes the extent of a chemical equilibrium from the point of view of the amount of reactants, 100% initially, and products.
Syllabus ref: R2.3.3Reactivity 2.3.3 - The magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent.
- Determine the relationships between K values for reactions that are the reverse of each other at the same temperature.
Guidance
- Include the extent of reaction for: K<<1, K<1 ,K = 1, K>1, K>>1.
Tools and links
- Reactivity 3.1 - How does the value of K for the dissociation of an acid convey information about its strength?
Equilibrium position
This is defined as the state of the reaction mixture, with respect to the reactants and products. The equilibrium law gives us a measure of the position of equilibrium.
The equilibrium law expression: 
For the general reaction: xA ⇋ yB + zC
Shows us that as the product concentration increases so does the value of Kc. Hence, for a reaction that lies well to the left hand side at equilibrium, the value of Kc is very small. Conversely, for a reaction that proceeds almost to completion and lies well to the right hand side, the value of Kc is very large.
We can assess the position of equilibrium by considering the value of Kc.
If a reaction proceeds nearly to completion, then the concentrations of the products is much larger than the concentration of the reactants and the value of the equilibrium constant is very large (much greater than 1).
If the reaction only makes a small quantity of the products at equilibrium, then the value of the equilibrium constant is very small (much less than 1)
| Value of Kc | position of equilibrium | reaction |
|---|---|---|
| << 1 | almost entirely reactants | almost none |
| >> 1 | almost entirely products | proceeds virtually to completion |
| ≈ 1 | roughly in the centre | both reactants and products present |
The equilibrium constant
By definition, when a system is in a state of equilibrium, the concentration of the components do not change. This condition, the equilibrium condition, can be arrived at from either direction, forwards or backwards.
It stands to reason, therefore, that the value of the equilibrium constant depends on the direction of the equilibrium under consideration. When we view a reversible reaction from the reverse direction, then reactants become producs, and vice-versa. For example: if we are dealing with a simple equilibrium situation where A ⇋ B, then the value of kc = [B]/[A].
For an equilibrium where [A] = 2 mol dm-3 and [B] = 1 mol dm-3, then kc = 1/2.
However, if we consider the equilibrium to be B ⇋ A, in other words, looking at the reverse reaction. Then kc = 2/1.
How can we prevent confusion, as the two values for the equilibrium constant are clearly not the same? We must make it clear from which direction the equilibrium constant is being determined, and if necessary state "forward" or "back" reactions.
The relationship between the values of the equilibrium constant for forward and back reactions is very simple. It is a reciprocal (inverse) relationship
Kc(forward) = 1/kc(backwards)
Factors affecting Kc
The equililbrium constant is ONLY affected by the temperature at which equilibrium is established.
An equilibrium will change its position towards the side of endothermic change if the temperature is increased. This is in accordance with Le Chatelier, as the system is responding to remove the extra energy that has been supplied. It does so by converting heat energy to chemical energy (thus reducing the heat).
Any change in Kc depends on the direction of endothermic change. If the equilibrium is endothermic in the forward direction, then increasing the temperature increases the products and the value of Kc. The opposite is true for an exothermic forward reaction.
|
Example: How does an increase in the temperature of the following equilibrium affect the value of the equilibrium constant? A(g) + B(g) ⇋ yC(g) + zD(g) ΔH = -150 kJ The reaction is exothermic (negative enthalpy change) in the forward direction, therefore increasing the temperature drives the reaction in the reverse direction, increasing the reactant concentration and reducing the product concentration. The vaue of Kc decreases correspondingly. |
The units of Kc
By units, we mean the dimensions of the equilibrium constant. The value of the equilibrium constant depends on the specific equation to which it applies and so do its units.
To find the units of any quantity that is derived from an equation it is simply a process of introducing the units for each of the equations components and then can cancelling down to arrive at the smallest possible unit .
|
Example: Find the units of the equilibrium constant for the equilibrium: A + B ⇋ yC + zD C + D The equilibrium constant is given by the equilibrium law equation
Substitute in the units for each of the components in the equation: It shoiuld be pretty clear that the units on the top of the sum are equal to the units on the bottom and so they cancel out:
|
Worked examples
Q724-01 For a reaction which goes to completion the equilibrium constant, Kc, is:- >>1
- <<1
- =1
- =0
|
The equilibrium law shows the concentration of the products as the numerator (on the top of the equation) and the reactants as the denominator (on the bottom), if the reaction goes to completion there are only reactants in the mixture and therefore the number on the top will be big and the number on the bottom is small giving an enormous value for Kc. Correct response = A |
Q724-02 Which statement is true about chemical reactions at equilibrium?
- The forward and backward reactions proceed at equal rates
- The forward and backward reactions have stopped
- The concentrations of the reactants and products are equal
- The forward reaction is exothermic
|
Equilibrium means the state at which the forward and the reverse reaction are at equal rate and the composition of the componenets remains unchanged. Correct response = A |
Q724-03 The position of a reversible reaction initially at equilibrium is shifted to the right until it reaches a new equilibrium situation. Which statement must be true for the reaction when the new position of equilibrium is reached?
- The rate of the forward reaction is greater than the rate of the reverse reaction.
- The concentration of reactants and products do not change
- The concentrations of reactants and products are equal
- The value of kc is greater than 1
|
Equilibrium means the state at which the forward and the reverse reaction are at equal rate and the composition of the components remains unchanged. In choice B the concentration of the components do not change. |
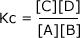
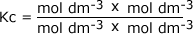
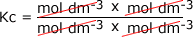 leaving the value of Kc dimensionless, i.e. it has no units.
leaving the value of Kc dimensionless, i.e. it has no units.