Higher-level only
The equilibrium constant measures the extent of a chemical reaction. At equilibrium there is no tendency for the reversible reaction to go forwards or backwards. Gibbs free energy change is zero. This section explores the link between K, the equilibrium constant and ΔG, Gibbs free energy change.
Syllabus ref: R2.3.7Reactivity 2.3.7 - The equilibrium constant and Gibbs energy change, ΔG, can both be used to measure the position of an equilibrium reaction. (HL)
Guidance
- Calculations using the equation ΔG⦵ = −RT lnK. The equation is given in the data booklet.
Tools and links
- Reactivity 1.4 - How can Gibbs energy be used to explain which of the forward or backward reaction is favoured before reaching equilibrium?
Gibbs free energy and entropy
Gibbs free energy is related to the entropy of the entire universe. It is a measure of the amount of chemical potential energy of a system that is available to do useful work. In doing so, the entropy of the universe would increase and thermodynamic feasibility of the process is confirmed.
Consider a reversible reaction from the point of view of the reactants, i.e. the forward reaction. If it is to be possible, then Gibbs free energy change must be negative. However, once equilibrium is attained the forward reaction is no longer possible and Gibbs free energy for a further hypothetical reaction must be positive.
The same argument can be applied to the reverse reaction. Initially, Gibbs free energy change is negative, but once equilibrium is attained it would become positive for a further hypothetical reaction.
Hence, at equilibrium the Gibbs free energy change in either direction is positive, i.e. no reaction is possible under the ambient conditions. At equilibrium, Gibbs free energy chage for any possible change to the system is positive. At this point, the entropy of the universe (and hence the system) is at a maximum and any further change would reduce it - fundamentally impossible.
To summarise, Gibbs free energy at equilibrium is zero, equilibrium represents a Gibbs free energy well, from which any further change is impossible (under the current conditions)
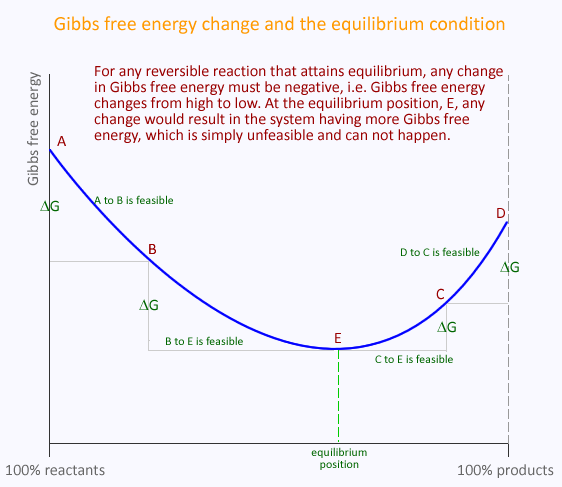
Gibbs free energy and the equilibrium constant
It is possible to relate the Gibbs free energy change of the initial hypothetical reaction in an equilibrium to the value of the equilibrium constant for the same reaction.
The formula is given in the data booklet:
ΔG = -RTlnk
Where: R is the universal gas constant, T is the absolute temperature and k is the equilibrium constant. "ln" means the natural logarithm (the number expressed as an exponent of the natural number base 'e', 2.718).
Determine an equilibrium constant.
Given that the value for Gibbs free energy change = 60 kJ mol-1, determine the value of the equilibrium constant at 300K
60 kJ = 6.0 x 104 J
R = 8.31 J K-1 mol-1
T = 300
6.0 x 104 = 8.31 x 300 (lnk)
lnk = 24.1, k = e24.1
k = 2.83 x 1010
The large value of the equilibrium constant, k, shows us the the equilibrim position lies almost totally on the side of the products, i.e. the reaction goes almost to completion.
Worked examples
Q722-01 Consider the endothermic reaction below:5CO(g) + I2O5(g) ⇋ 5CO2(g) + I2(g)
According to Le Chatelier's principle, which change would result in an increase in the amount of CO2?
- Increasing the temperature
- Decreasing the temperature
- Increasing the pressure
- Decreasing the pressure
|
As it is an endothermic reaction in the forward direction an increase in temperature pushes the equilibrium to the side of the products. There is no change in the number of moles and so the equilibrium is unaffected by pressure change. The correct response is A. |
Q722-02 The compounds N2O4 and NO2 produce an equilibrium mixture according to the equation below:
N2O4 (g) ⇋ 2NO2(g) ........ ∆H = +57.2 kJmol-1
An increase in the equilibrium concentration of NO2 can be produced by increasing which of the following factors:
- I. Pressure
- II. Temperature
|
Increasing the temperature pushes the reaction in the direction of ENDOthermic change, ie to the right hand side. Increasing the pressure pushes the reaction in the direction of the fewer number of moles of gas, ie to the left hand side in this case. |
Q722-03 For the following system at equilibrium, which change will shift the position of equilibrium to the right?
CH3COOH(l) + C2H5OH(l) ⇋ CH3COOC2H5(l) + H2O(l) ..... ∆H < 0
- Adding a catalyst
- Increasing the pressure
- Removing water
- Increasing the temperature
|
Remove from one side of an equilibrium and you pull the reaction towards that same side. If you remove one of the reacting components of the REVERSE reaction, then the rate of the reverse reaction becomes less than that of the forward reaction. The system then reacts faster in the forward direction, making more of the right hand side. |
Q722-04 Which change will shift the position of equilibrium to the right in this reaction?
N2(g) + 3H2(g) ⇋ 2NH3(g) ... ∆H = - 92 kJ
- Increasing the temperature.
- Decreasing the pressure.
- Adding a catalyst.
- Removing the ammonia from the equilibrium mixture.
|
The sign of ∆H is negative therefore it is exothermic in the forward direction. Increasing temperature favours the direction of ENDOthermic change ie to the left hand side in this case. II does not go to the right hand side. Decreasing the pressure forces the equilibrium to move towards the side of greater moles of gas to re-establish the equilibrium concentrations. Catalysts do NOT affect equilibria. Removing ammonia disturbs the equilibrium concentrations and the reaction responds by making more ammonia to re-establish the equilibrium concentration proportions - this is the correct response. |
Q722-05
2H2O(l) ⇋ H3O+(aq) +OH-(aq)
The equilibrium constant for the reaction above is 1.0 x 10-14 at 25ºC and 2.1 x 10-14 at 35ºC. What can be concluded from this information?
- [H3O+] decreases as the temperature is raised
- [H3O+] is greater than [OH-] at 35ºC
- Water is a stronger electrolyte at 25ºC
- The ionization of water is endothermic.
|
Kc has a larger value at the higher temperature. Thus the temperature increases and the proportion of products increases. Temperature increase favours the direction of endothermic change therefore this reaction is endothermic in the forward direction. |
Q722-06 Which change increases the amount of NH4+ in the below reaction?
NH3(g) + H2O(l) ⇋ NH4+(aq) + OH-(aq).... ∆H > 0
- Decreasing the temperature
- Decreasing the pressure
- Removing water
- Adding an acid
|
The sign of ∆H is positive (it is greater than 0) therefore it is endothermic in the forward direction. Increasing temperature favours the direction of ENDOthermic change ie to the right hand side in this case. Decreasing the temperature will move the equilibrium position to the left hand side. Decreasing the pressure forces the equilibrium to move towards the side of greater moles of gas to re-establish the equilibrium concentrations, in this case the left (notice the state symbols) Removing water disturbs the equilibrium concentrations and the reaction responds by making more water to re-establish the equilibrium concentration proportions. This pulls the reaction to the left to restore the value of Kc. Adding acid reacts with the OH- ions from the right hand side disturbing the equilibrium proportions that are then re-established by the reaction moving to the right hand side making more NH4+. |
Q722-07 Consider the equilibrium reaction:
4NH3(g) + 3O2(g) ⇋ 2N2(g) + 6H2O(g) ΔH = -1268 kJ
Which change will cause the reaction to shift to the right?
- Increase the temperature
- Decrease the volume of the container.
- Add a catalyst to speed up the reaction.
- Remove the gaseous water by allowing it to react and be absorbed by KOH.
|
The reaction is exothermic in the forward direction, thus increasing the temperature drives the equilibrium in the reverse direction. Decreasing the volume increases the pressure. The equilibrium has 7 moles of gas on the left and 8 moles of gas on the right, hence increasing the pressure drives the reaction to the left hand side. Adding a catalyst has no effect on the position of equilibrium. Removing the gasous water disturbs the equilibrium and the system must respond by making more water, i.e. by moving to the right to re-establish the equilibrium proportions. |
Q722-08 Which changes will shift the position of equilibrium to the right in the following reaction?
2CO2(g) ⇋ 2CO(g) + O2(g)
- I. adding a catalyst
- II. decreasing the oxygen concentration
- III. increasing the volume of the cylinder
- I and II only
- I and III only
- II and III only
- I, II and III
|
I - catalysts have no effect on equilibrium. II - decreasing the oxygen concentration pulls the reaction to the right to re-establish the equilibrium proportions. II - increasing the volume decreases the pressure. There are two moles of gas on the left and three moles of gas on the right, hence a decrease in pressure moves the reaction towards the side of the most moles (the right) to re-establish the equilibrium proportions. Corrct response II and III only |
Q722-09 The reaction below is an important step in the production of sulfuric acid. An increase in which of the following, will increase the ratio of SO3(g) to SO2(g) at equilibrium?
2SO2(g) + O2(g) ⇋ 2SO3(g) ΔH = -197 kJ
- Pressure only
- Temperature
- Both pressure and temperature
- Neither pressure nor temperature
|
Increase pressure moves the reaction in the directin of fewer moles, in this case to the right. This increases the ratio of SO3(g) to SO2(g) at equilibrium. Increased temperature moves the position of equilibrium towards the side of endothermic change. in this case the left. This reduces the ratio of SO3(g) to SO2(g) at equilibrium. Correct response A |
Q722-10 The equation for a reaction used in the manufacture of nitric acid is:
4NH3(g) + 5O2(g) ⇋ 4NO(g) + 6H2O(g) ΔH = -900 kJ
Which changes occur when the temperature of the reaction is increased?
|
Position of equilibrium
|
Value of Kc
|
|
|
A.
|
shifts to the left
|
increases
|
|
B.
|
shifts to the left
|
decreases
|
|
C.
|
shifts to the right
|
increases
|
|
D.
|
shifts to the right
|
decreases
|
|
When the temperature is increased the position of equilibrium moves towards the direction of endothermic change, in this case it shifts to the left. The value of Kc decreases as there is less product (right hand side) and more reactants (left hand side) at equilibrium. Correct response B |