Higher-level only
There are many different types of indicators. Although they may all be used for pH detection they are not all suitable for the same purpose.
Syllabus ref: R3.1.15Reactivity 3.1.15 - An appropriate indicator for a titration has an end point range that coincides with the pH at the equivalence point. (HL)
- Identify an appropriate indicator for a titration from the identity of the salt and the pH range of the indicator.
Guidance
- Distinguish between the terms “end point” and “equivalence point”.
Tools and links
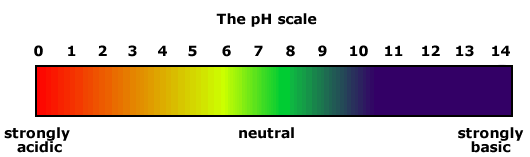
Universal indicator
Universal indicator is a mixture of coloured compounds, which is used for simple testing of solutions. It is of no use for titrations as there are several colour changes that take place over a variety of pH values.
Universal indicator is also available in the form of test paper. This consists of strips of paper impregnated with universal indicator solution and dried. It may be used to test solutions or gases. To test gases the paper must be dampened previously wth a drop of water and then held in the gas.
Litmus
Litmus is a vegetable based dye that was used in schools before the advent of universal indicator. It has a simple colour scheme, in acids it turns red and in bases it turns blue, however the mid-point is not sharply defined and is unsuitable for titrations.
It is usually used in the form of paper impregnated with the litmus dye. This comes in two varieties, the red paper that is used to test for bases and the blue litmus paper that is used to test for acids.
Litmus paper when previously dampened can also be used to test for the acidity of gases.
Phenolphthalein
Phenolphthalein is an organic indicator with a pKa value of 9.3. It is red in bases and colourless in acids.
Although it has a complicated structure, the good news is that you don't need to know it! In basic solution the molecule loses a hydrogen ion and becomes a negative ion. This can be represented by the much simpler equation:
HIn ⇋ H+ + In-
The molecular form of phenolphthalein is represented as HIn and the ionic form as In-.
When base is added it removes the hydrogen ions from the equilibrium and the equation moves to the right hand side. We see the colour of the anion, i.e. red.
One slight complication of phenolphthalein is that the colour tends to fade as new products are formed on standing. In titrations the readings at the end point should be taken fairly quickly if adding base to acid.
Phenolphalein is the indicator of choice when titrating weak acids with strong bases.
Ethanoic acid with sodium hydroxide
Methyl orange
Methyl orange is an acid base indicator that turns red in acidic solution and yellow in base, the mid-point colour is orange. Its pKa value is 3.4.
The fact that its pKa lies in the acidic region of the pH scale makes methyl orange useful for titrations involving weak bases and strong acids, such as ammonia solution and hydrochloric acid. The equivalence point of this titration also lies in the acidic region and so the colour change is marked when the mixture passes through the neutralisation point.
 |
 |
 |
|
Methyl orange in acidic solution
|
Methyl orange at the equivalence point
|
Methyl orange in base solution |
Bromothymol blue
Bromothymol blue is an acid base indicator which turns yellow in acid solution and blue in base. It has a pKa of 7.0 and consequently is used for titrations involving strong acids and strong bases.
Choice of indicator summary
As discussed above, the indicator must be chosen to suit the titration being performed.
Titrations involving strong acids and bases can be performed using any indicator as the pH change at the equivalence point is large (from 3 to 11 or vice versa) - most indicators operate within this range.
Titrations involving strong acids and weak bases have an equivalence point in the acidic region of the pH scale. The choice of indicator will depend on the actual expected pH at the equivalence point. It makes sense to select an indicator with a pKa right in the middle of the pH change at the equivalence point. In practice, there are few indicators in common use. For strong acids and weak bases methyl orange is the indicator of choice.
Worked examples
Q884-01 Which 0.1 M solution will turn phenolphthalein pink?- HBr(aq)
- CO2(aq)
- LiOH(aq)
- CH3OH(aq)
|
Phenolphthalein indicator changes to pink in basic solutions (above pH 8.3) The only base in the list is lithium hydroxide LiOH - Response C |
Q884-02 The indicator ravishing red has a color change from yellow to red in the pH range 6.5- 7.5. This indicator would be used to titrate
- a weak acid with a strong base
- a strong acid with a weak base
- a weak acid with a weaker acid
- a strong acid with a strong base
| The pH range of the indicator falls at the neutral point pH7
the equivalence point of a strong acid and strong base is at pH 7 and so this indicator is suitable for a titration of this kind - Response D |
Q884-03 Which solution will change red litmus to blue?
- HCl(aq)
- NaCl(aq)
- CH3OH(aq)
- NaOH(aq)
|
Litmus is an indicator of vegetable origin. It used to be used frequently in school level chemistry but has largely been superceded by universal indicator. The colour produced by litmus is red in acids and blue in alkali solutions. |
Q884-04 When conducting analyses of substances that are weak acids by titrating solutions with a standardised strong base, the end-point indicator is chosen so that
- its color change occurs around the neutralization pH of 7.00.
- its color change occurs when the pH is about the same as the pKa of the weak acid.
- its color change occurs at a pH that is more basic than pH = 7.00.
- its color change occurs at a pH that is the same as that of the standardised base solution.
|
The colour change should be in the basic range, i.e. greater than pH = 7. |
Q884-05 An acid-base indicator, HIn, dissociates according to the following equation:
HIn(aq) ⇋ H+(aq) + In-(aq)
colour A colour
B
Which of the following statement(s) about the indicator is/are correct?
- I. In a strongly acidic solution colour B would be seen
- II. In a neutral solution the concentrations of HIn(aq) and In-(aq) must be equal
- III. It is suitable for use in titrations involving weak acids and weak bases
- I only
- II only
- III only
- None of the above
|
Strongly acidic solutions have a high value for [H+] and the equilibrium is driven to the left hand side revealing colour A, hence the first statement is incorrect. The second statement is also incorrect, as the equimolar amounts occur when the pH = Ka of the indicator, not necessarily in a neutral solution. Statement C is incorrect as weak acids and weak bases cannot be titrated. The correct response is D, none of the above. |
Q884-06 Which neutralisation reaction could use phenolphthalein (Ka = 9.3) and not methyl orange (pKa = 3.7) as an indicator?
- NaOH(aq) and HNO3(aq)
- NH3(aq) and CH3COOH(aq)
- NaOH(aq) and CH3COOH(aq)
- NH3(aq) and HNO3(aq)
|
Phenolphthalein changes colour in the basic region of the pH scale. Titrations in which the point of inflexion occur both sides of pH = 9.3 are those performed with a strong base and a weak acid. The correct response is NaOH(aq) and CH3COOH(aq) |
Q884-07 Which solution will change methyl orange to yellow?
- HCl(aq)
- NaCl(aq)
- CH3OH(aq)
- NaOH(aq)
|
Methyl orange is yellow in basic solution above pH 3-4 - Response D |
Q884-08 Which statement about indicators is always correct?
- The mid-point of an indicator's colour change is at pH=7
- The pH range is greater for indicators with higher pKa values
- The colour red indicates an acidic solution
- The pKa value of the indicator is within its pH range
|
Indicators change colour when there is an equal concentration of both the ionic and the molecular form. This occurs roughly when the pH = pKa. Hence the pKa value of the indicator must be within its pH range. |