Higher-level only
Cell potentials refer to the voltage that is created between two half cells when they are connected via an external circuit.
Syllabus ref: R3.2.13Reactivity 3.2.13 - Standard cell potential, E⦵cell, can be calculated from standard electrode potentials. E⦵cell has a positive value for a spontaneous reaction. (HL)
- Predict whether a reaction is spontaneous in the forward or reverse direction from E⦵ data.
Guidance
Tools and links
Standard cell potential
When two half cells are combined using an external circuit and salt bridge, a potential difference arises due to the different electrode potentials of the two half cells.
The cell with the more negative potential will attempt to push electrons around the external circuit towards the less negative half cell. The measured potential (using a high resistance voltmeter) is called the cell potential - it is equal to the DIFFERENCE between the electrode potentials of the two half cells.
The direction of (attempted) flow is from the cell with the most negative potential to the cell with the least negative (most positive) potential.
Example:In the following cell made up of two half cells the standard electrode potential of zinc = -0.76V and that of copper is +0.34V
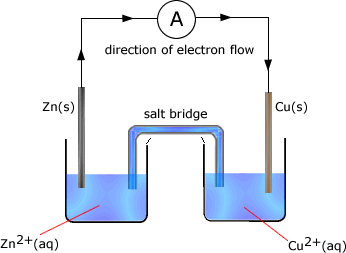
The cell potential is the difference between the standard electrode potential values
= -0.76 - +0.34 = -1.10V
The zinc half cell has the most negative potential and so the direction of electron flow would be from the zinc half cell to the copper half cell.
Reactions occuring in the half cells
As the zinc half-cell releases electrons then..
Zn ⇋ Zn2+ + 2e
As the copper half-cell accepts electrons then..
Cu2+ + 2e → Cu
Adding these two reactions gives the overall cell reaction as:
Zn + Cu2+ → Zn2+ + Cu
cell potential = 1.10 V
Spontaneity of a reaction
Spontaneous in this context does not mean 'happens immediately'. It just means that the reaction is possible in terms of the energetics (thermodynamics) of the process. Another word that could be used is 'feasible'.

Feasible reactions can be identified from the relative electrode potential values of the reacting species.
Any combination of an oxidising agent with a reducing agent, where the difference in electrode potentials is greater than 0.3 V. Basically something on the left will react with something higher up from the right hand side.
The equations for the reactions can be constructed by first balancing the number of electrons in each half-equation and then adding the half-equations together.
Prediction of spontaneity
Electrode potentials for redox systems can be used to predict whether or not a proposed reaction could proceed. Take the reaction between copper ions and zinc metal. The two half-equations are:
Zn2+ + 2e ⇋ Zn
Cu2+ + 2e ⇋ Cu
The zinc half-equation is reversed and added to the copper half-equation:
Cu2+ + Zn ⇋ Cu + Zn2+
This is the proposed reaction. By inspection we can see that if the reaction proceeds the copper ions are going to be reduced (they have electrons added) and the zinc atoms are going to be oxidised (they have electrons removed).
The zinc is said to be the oxidised state (the part getting oxidised) and the copper ions the reduced state (the part getting reduced).
To find spontaneity we apply the equation:
Eo (species that gets reduced) - Eo (species that gets oxidised) = Eo (cell potential)
- If the E (cell potential) is negative then there is no reaction between these species
- If the value given lies between 0 and 0.3 then an equilibrium is established
- If the value for E (cell) is greater than +0.3 then reaction between these species is spontaneous
As:
Cu2+ + 2e ⇋ Cu Eo = +0.34V
and
Zn2+ + 2e ⇋ Zn Eo = -0.76V
then
E = E (red) - E (ox)
E = +0.34 - (-0.76)V
E = + 1.10V
The answer is positive and greater than 0.3V therefore the reaction is spontaneous - zinc reacts with copper ions.
Example: Is the reaction between dichromate ions and tin 2+ ions spontaneous?
Cr2O72-(aq) + 14H+(aq) + 6e- → 2Cr3+(aq)
+ 7H2O(l) (Eo = + 1.33 V)
Sn4+(aq) + 2e- → Sn2+(aq) (Eo = + 0.55 V)
Cr2O72-(aq) + 14H+(aq) + 3Sn4+(aq) → 2Cr3+(aq) + 7H2O(l) + 3Sn2+(aq)
Spontaneity is decided by applying E = E (red) - E (ox)
In this case the Cr2O72- is reduced and the Sn2+ oxidised therefore
E = 1.33 - 0.55 = 0.78 V
This is a positive value greater than 0.3, therefore the reaction is spontaneous.
Feasible reactions can be identified by any combination of an oxidising agent with a reducing agent, where the difference in electrode potentials is greater than 0.3 V. Basically something on the left will react with something higher up from the right hand side.
The equations for the reactions can be constructed by first balancing the number of electrons in each half-equation and then adding the half-equations together.
Example: Construct the equation for the reaction between dichromate ions and tin 2+ ions
The dichromate half-equation is:
1Cr2O72-(aq) + 14H+(aq) + 6e- → 2Cr3+(aq) + 7H2O(l) (Eo
= + 1.33 V)
And the tin 2+ half-equation is:
2Sn4+(aq) + 2e- → Sn2+(aq)
(Eo
= 0.55 V)
By inspection we can see that the dichromate equation needs six electrons on the left hand side whereas the tin 2+ equation has only two electrons on the right hand side. We must, then, multiply the tin 2+ equation by three before adding them.
| 1 | Cr2O72-(aq) + 14H+(aq) + 6e- ⇌ 2Cr3+(aq) + 7H2O(l) | |
| 2 | Sn4+(aq) + 2e- ⇌ Sn2+(aq) | multiply 2 by 3 |
| 3 | 3Sn4+(aq) + 6e- ⇌ 3Sn2+(aq) | |
| add 1 and 3 | ||
| 4 | Cr2O72-(aq) + 14H+(aq) + 3Sn4+(aq) → 2Cr3+(aq) + 7H2O(l) + 3Sn2+(aq) |
Whether or not the reaction will be spontaneous is decided by applying E = E (red) - E (ox)
In this case the Cr2O72- is reduced and the Sn2+ oxidised therefore
E = 1.33 - 0.55 = 0.78 V
This is a positive value greater than 0.3 therefore the reaction is spontaneous.
Exceptions
1 These standard electrode potential values
refer to standard
conditions i.e. 1.0 Molar concentrations at 25oC and atmospheric
pressure. If these conditions change then so does the electrode potential.
In other words, for calculating cell potential, or spontaneity of reaction,
it is important to understand that variations occur.
For example, according to standard electrode potentials, manganese IV oxide will not react spontaneously with hydrochloric acid, however this is the standard preparation of chlorine in the laboratory.
| MnO2(s) + 4H+(aq) + 2e- ⇌ Mn2+ + 2H2O(l) | E |
|
| Cl2(g) + 2e- ⇌ 2Cl-(aq) | E |
|
| Predicting spontaneity, E = E(red) - E(ox) | = 1.23 - 1.36 | E = - 0.13 V |
| Negative value therefore no reaction!! |
In the lab preparation the manganese IV oxide is heated with the concentrated
HCl - these are not standard
conditions, the temperature is much greater than 25oC and
the concentration of the acid much greater than 1.0 mol dm-3. Under
these new conditions the reaction becomes spontaneous and proceeds at a comfortable
rate to collect the chlorine gas produced.
MnO2(s) + 4H+(aq) + 2Cl-(aq) → Mn2+ + 2H2O(l) + Cl2(g)
Worked examples
R3213-01 Given the standard electrode (reduction) potentials:- Cd2+(aq) + 2e → Cd(s) E
o= -0.40 V - Ag+(aq) + e → Ag(s) E
o= +0.80 V
What would be the Eo
for a cadmium-silver cell?
- 0.4 V
- 0.5 V
- 1.2 V
- 2.0 V
|
The cell potential is given by the difference between the standard electrode potentials of the component half-cells. E The negative sign is relatively meaningless as the cell potential difference is simply a magnitude. Therefore = 1.20 V. |
R3213-02 The standard electrode potentials for Al and Mn are given below:
- Al3+(aq) + 3e →
Al(s) E
o= -1.66 V - Mn2+(aq) + 2e →
Mn(s) E
o= -1.18 V
What is the potential of a cell prepared with these metals in contact with 1.0 mol dm-3 solutions of their ions?
- 0.22 V
- 0.48 V
- 2.84 V
- 3.43 V
|
1.0 mol dm-3 solutions are standard conditions. The cell potential is obtained by the difference between the two standard electrode potentials of the half-cells. Therefore standard cell potential = -1.66V - (-1.18) = 0.48V |
R3213-03 Use the data table to determine E
- -2.45 V
- -0.27 V
- +0.27 V
- +2.45 V
|
The difference between the standard electrode potential of the chlorine|chloride half cell and the bromine|bromide half cell is 1.36 - 1.07 = 0.27 V |
R3213-04 Consider these standard electrode potentials:
- Cu2+(aq) + 1e → Cu+(aq) E
o= +0.15 V - Cu+(aq) + 1e → Cu(s) E
o= +0.52 V
What is the standard cell potential when the two half cells are connected?
- -0.67V
- -0.37V
- +0.37V
- +0.67V
|
The difference between the standard electrode potential of the copper (I) |copper half cell and the copper(II) |copper(I) half cell is 0.52 - 0.15 = 0.37 V |
R3213-05 The standard electrode potentials of two metals are given below. Using this information what are the equation and cell potential for the spontaneous reaction that occurs?
- Tl+(aq) + e- →
Tl(s) E
o= -0.336 V - Cu2+(aq) + 2e- →
Cu(s) E
o= +0.339 V
- Tl+(aq) + Cu2+(aq) --> Tl(s) +
Cu(s) E
o= 0.003 V - 2Tl(s) + Cu2+(aq) --> 2Tl+(aq)
+ Cu(s) E
o= 0.675 V - 2Tl(s) + Cu2+(aq) --> 2Tl+(aq)
+ Cu(s) E
o= 1.011 V - Tl+(aq) + Cu(s) --> 2Tl(s) + Cu2+(aq)
E
o= 0.333 V
|
Using the values for electrode potentials the strongest oxidising
agent (left hand side, most positive E Therefore when Cu2+(aq) reacts with Ti(s), the Cu2+(aq) gets reduced and the Ti(s) gets oxidised. E = E(reduced species) - E(oxidised species) = 0.339 - - 0.336 = 0.675 V Hence the equation is: 2Tl(s)
+ Cu2+(aq) --> 2Tl+(aq) + Cu(s) E |
R3213-06 Draw a cell diagram for the cell formed by connecting the following standard half cells.
Ni(s)/Ni2+(aq) || Cd2+(aq)/Cd(s)
Given:
- Ni2+(aq) + 2e → Ni(s) E
o= -0.2 V - Cd2+(aq) + 2e → Cd(s) E
o= -0.4 V
Write an equation for the reaction in each half cell, identify the species which is oxidised and the oxidising agent.
Answer|
The species oxidised is Cd(s) and the species reduced is Ni2+(aq). |
R3213-07 An electrochemical cell is constructed from two half cells connected by a high resistance voltmeter. One half cell contains nickel in a solution of nickel nitrate and the other half cell contains silver in a solution of silver nitrate.
State the conditions that must be applied to the solutions for the measurements taken in the cell to be considered as standard. [2]
Outline how the two half-cells must be connected before any voltage readings can be made.[2]
Assuming that standard conditions apply use the values from the data table to calculate the potential of the cell. Write the shorthand notation for the cell including state symbols and give the equation for the reaction occurring in the cell. [2]
Answer
|
Standard conditions means that the concentrations of the solutions
must be 1.0 mol dm-3 and the temperature must be at 25 The two half-cells must have the solutions connected by a salt bridge and the external circuit must have a high resistance voltmeter. The cell potential is given by the difference between the two half-cell potentials = 0.80 - - 0.20 = 1.00 V The standard notation for the cell is: Ni(s)|Ni2+(aq)||Ag+(aq)|Ag(s) |
R3213-08 A half cell (A) is set up by placing a platinum electrode in a solution containing both Fe2+ and Fe3+ ions at a concentration of 1 mol dm-3. This half cell is then connected by means of a salt bridge to another half cell (B) containing an iron electrode in a 1 mol dm-3 solution of Fe2+ ions.
State the function of the salt bridge [1]
The two electrodes are connected externally. Use the data given to determine the cell potential. [2]
Give the redox reactions that occur in each half cell. [2]
State the direction of electron flow in the external circuit. [1]
Answer
|
The salt bridge is to connect the circuit and allow migration of ions to equalise charge in the half-cells. The standard cell potential is the difference between the standard electrode potentials of the two half cells = 0.77 - - 0.41 = 1.18 V In the most negative half-cell, electrons are produced by the strongest reducing agent, which in this case is Fe(s). The equation is: Fe(s) → Fe2+(aq) + 2e In the positive half-cell the electrons are used to reduce the best oxidising agent, which in this case is Fe3+(aq) according to the equation: Fe3+(aq) + 1e → Fe2+(aq) The electrons flow around the external circuit from the negative half-cell to the positive, i.e. from the Fe2+(aq)|Fe(s) half-cell to the Fe3+(aq)|Fe2+(aq) half-cell. |
R3213-09 The standard electrode potentials for three electrode systems are given below:
- Ti3+(aq) + 1e → Ti2+(aq) E
o= -0.37 V - Fe3+(aq) + 1e → Fe2+(aq) E
o= +0.77 V - Ce4+(aq) + 1e → Ce3+(aq) E
o= +1.45 V
Write an equation including state symbols for the overall reaction with the greatest cell potential. [2]
Answer|
The strongest oxidising agent (most positive E The strongest reducing agent (most negative, right hand side) is Ti2+(aq). The greatest cell potential is for the reaction between these two species. The equations involve the same number of electrons and can be added directly. Ce4+(aq) + Ti2+(aq) → Ce3+(aq) + Ti3+(aq) |
R3213-10 Calculate the cell potential of a cell made by connecting standard copper and zinc electrodes, given the following values for standard redox potentials.
- Zn2+(aq) + 2e → Zn(s) E
o= -0.76 V - Cu2+(aq) + 2e → Cu(s) E
o= +0.34 V
State the direction of electron flow in the external circuit when the cell produces current. Outline the changes occurring at the electrodes and in the solutions during the process [5]
Answer|
The cell potential is given by the difference between the two electrode potentials, in this case 0.34 - - 0.76 = 1.10 V. The Zn2+(aq)|Zn(s) half-cell is the negative provider of electrons and the Cu2+(aq)|Cu(s) half-cell is the positive acceptor of electrons. Hence electrons flow around the external circuit from the zinc to the copper. At the zinc (negative) electrode, zinc atoms are dissolving as zinc ions, leaving behind electrons to flow to the copper electrode. The process at the electrode is:
At the copper (positive) electrode, copper ions pick up electrons to become copper atoms. The process at the electrode is:
During the course of the reaction the zinc (negative) electrode loses mass and the copper (positive) electrode gains mass. The zinc ion solution gets more concentrated and the copper ion solution becomes less concentrated. |
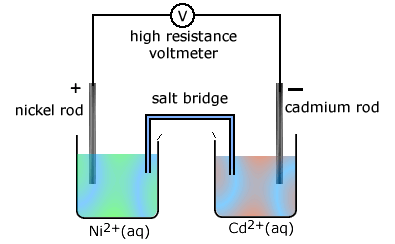 The stongest oxidising agent (left hand side, most positive) is Ni2+(aq)
and the best reducing agent (most negative, right hand side) is Cd(s).
The reaction proceeding in the cell is:
The stongest oxidising agent (left hand side, most positive) is Ni2+(aq)
and the best reducing agent (most negative, right hand side) is Cd(s).
The reaction proceeding in the cell is: