Higher-level only
Electroplating, or electrodeposition, is the process of applying a layer of metal to the surface of another metal. It is a variation on the theme of electrolysis. There are many applications of this process in use in the modern world.
Syllabus ref: R3.2.16Reactivity 3.2.16 - Electroplating involves the electrolytic coating of an object with a metallic thin layer. (HL)
- Deduce equations for the electrode reactions during electroplating.
Guidance
Tools and links
- Tool 1 - How is an electrolytic cell used for electroplating?

The purification of copper
The participating electrode effect discussed in the previous section can be put to good use in the purification of copper. Impure copper can be used as the anode in an electrolysis cell and pure copper gets deposited at the cathode. The concentration of copper ions in the electrolyte remains unchanged.
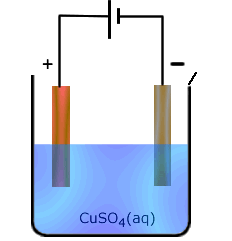 |
The reaction at the anode: Cu(s) - 2e → Cu2+(aq)
Reaction at the cathode Cu2+(aq) + 2e → Cu(s) |
Electroplating
Any metal lower than hydrogen in the electrochemical series, i.e. with a positive electrode potential, can be deposited at the cathode during electrolysis. This means that these metals can be used to electroplate other substances. The requirements for successful electroplating are quite stringent in that the deposit of metal often fails to adhere successfully to the material of the cathode, unless the conditions are optimised.
Suitable electroplating metals include:
- Copper
- Chromium
- Nickel
- Silver
Chrome plating
Chromium is used in many areas as a decorative coating due to its bright silver colour and shine. There are two electrolytic processes used for chrome plating, which differ only in the electrolyte used. There are advantages and disadvantages in both processes.
In the hexavalent chromium process, the electrolyte used is chromium(VI) oxide dissolved in sulfuric acid. This produces a highly acidic and toxic mixture. In the trivalent chromium process the electrolyte is chromium(III) sulfate or chromium(III) chloride.
Both processes involve three phases:
- 1 Activation
- 2 Chromium electroplating
- 3 Rinsing
The activation stage involves etching and cleaning the surface of the metal to be electroplated, so that the layer of chromium adheres tightly to the surface. It typically takes place in a bath of chromic acid, using reverse current to remove surface impurities.
 The electrolytic
stage the current and temperature are carefully controlled to give the coating
characteristics required. In the chromium(III) process additives must be used
to prevent oxidation taking place at the anode.
The electrolytic
stage the current and temperature are carefully controlled to give the coating
characteristics required. In the chromium(III) process additives must be used
to prevent oxidation taking place at the anode.
The main disadvantages of the hexavalent process is its cost and toxicity (The film Erin Brockovich is a true story about a woman pursuing compensation from a company that polluted the land with hexavalent chromium causing health problems and morbidity in the local population). The final product is not toxic, as the rinsing procedure leaves only pure chromium.
The disadvantage of the trivalent process is that the colour of the chrome plate is not always up to customer expectations. However, this problem has been resolved by the use of certain additives. The plating is generally more even than the hexavalent process.
Worked examples
Q973-01 Electroplating processes usually involve depositing a metal from a solution of some salt, or ion of the metal. This deposition occurs- at the cathode of the cell.
- at the anode of the cell.
- when the metal is oxidized.
- when electrons are lost by the metal.
|
Metal ions always have a positive charge and deposit on the negative electrode, the cathode in electrolysis. |
Q973-02 In the electrolytic refining of copper, an electric current is passed through a cell containing a pure copper electrode, an electrode of impure copper, and an aqueous solution containing copper cations. As metallic copper is removed from the impure electrode and redeposited on the pure copper electrode other metallic impurities are left behind, if the potential of the cell is adjusted properly. In this process, the impure copper electrode is called the..?
- anode.
- cathode.
- reference electrode.
- indicating electrode.
|
The process occurring at the impure copper electrode is:
This occurs at the anode. |
Q973-03 A metallic object is electroplated with copper using a solution of copper(II) sulfate. Which statement is correct?
- The positive electrode increases in mass
- The concentration of Cu2+ ions in the solution decreases
- Reduction occurs at the positive electrode
- The reaction occurring at the negative electrode is Cu2+ + 2e → Cu
|
The positive electrode decreases in mass as copper ions leave into the solution. The concentration of copper ions in solutionremains the same as ions are being added and removed at the same rate. The positive electrode pulls electrons off the atoms according to the equation: Cu(s) - 2e → Cu2+(aq) The reaction occurring at the negative electrode is Cu2+ + 2e → Cu |
Q973-04 Calculate the mass of pure copper deposited at the cathode during the purification of an impure copper anode if 10A are passed through a solution of copper(II) sulfate(aq) for 1 hour. [Cu = 63.5]
Answer
|
Charge passed = It = 10 x 60 x 60 = 36,000 Coulombs Faradays of charge = 36,000/96,500 = 0.373 F = 0.373 moles of electrons Equation for the deposition: Cu2+(aq) + 2e → Cu(s) Hence 0.373 moles of electrons deposits 0.373/2 moles of copper = 0.187 moles Cu Therefore mass of Cu = 0.187 x 63.5 = 11.8 g |
Q973-05 Two copper strips X and Y are placed in a solution of copper(II) sulfate and electrolysed for a certain time. X is attached to the negative terminal of the battery and Y to the positive terminal. X is then removed, dried and weighed. State and explain what would happen to the mass of X. [3]
Give two ways in which the change in mass of X could be increased. [2]
Answer|
At the negative electrode copper is deposited according to the equation:
so the mass of the cathode increases. The mass could be increased by electrolysing for longer, or by using a higher current. |
Q973-06 Electroplating may be used to coat one metal with another metal.
a) Identify the three factors affecting the amount of metal discharged during
electroplating [3]
b) Explain why electrolysis of zinc sulfate solution is not used for coating
with zinc metal. [2]
|
a) The three factors are current, time and charge on the metal ion. b) zinc has a redox potential of -0.76 V. The electrode potential of hydrogen is 0 V, therefore hydrogen gas is preferentially released instead of zinc in aqueous solution. |
Q973-07 Electrolysis of an aqueous solution of copper(II) sulfate can be carried out using copper electrodes instead of inert electrodes. Describe the difference in the observations at each electrode and the electrolyte in the two cases.
Answer
|
The anode undergoes the following reaction:
This means that the mass of the anode decreases as copper metal turns to copper ions in solution The cathode undergoes the following reaction:
This means that the mass of the cathode increases as copper metal is deposited. In the solution, the copper ions are created at the anode, but they are used up at the cathode. Overall there is no change in concentration of copper ions in solution. |
Q973-08 A current of 0.2 amperes passing for 5 hours through a solution of gold ions deposits a mass of 1.45g of gold at the cathode. Calculate the charge on the gold ions. [Ar of gold = 197]
Answer
|
Faradays of charge = Coulombs/96500 = 0.0373 moles of electrons. Moles of gold deposited = mass/RAM = 1.45/197 = 0.0124 moles Thus 0.0373 moles of electrons deposit 0.0124 moles of gold, i.e. 1 mole of gold requires 3 moles of electrons. Therefore the charge on a gold ion = 3+ |
Q973-09 Calculate the mass of copper deposited on a copper cathode by electrolysis of copper(II) sulfate solution, if the same current deposited 0.9g of silver from silver nitrate solution in the same period of time. [Ag = 108 Cu = 63.5]
Answer
|
0.9g of silver represents 0.9/108 moles = 0.00833 moles Silver gets deposited according to the equation:
However, copper requires twice as many moles of electrons for the same moles of copper:
Hence, moles of copper produced = 0.00833/2 = 0.00417 moles Mass = moles x RAM = 0.00417 x 63.5 = 0.265g (3 sig figs) |
Q973-10 A current of 0.75 amperes passes through 250cm3 of 0.25 mol dm-3 copper(II) sulfate solution. Calculate how long it will take for all of the copper to be deposited at the cathode.
Answer
|
250cm3 of 0.25 mol dm-3 copper(II) sulfate solution contains 0.25 x 0.25 moles of copper = 0.0625 moles Copper deposition occurs according to the equation:
1 mole of copper is produced per 2 moles of electrons. Hence 0.0625 moles of copper requires 0.0625 x 2 moles of electrons (Faradays) = 0.125 F 0.125 Faradays = 12063 Coulombs = current x time (seconds) Thus time required = 12061.5/0.75 = 16083 s = 268 mins 33s = 4 hours 28 mins 33 seconds |