Standard level
Electrochemical cells are made up of two half-cells which, when connected together, allow an electrical current to flow around an external circuit. They are an essential source of electrical energy in the modern world, powering watches, mp3 players etc..
Syllabus ref: R3.2.5Reactivity 3.2.5 - Oxidation occurs at the anode and reduction occurs at the cathode in electrochemical cells.
- Identify electrodes as anode and cathode, and identify their signs/polarities in voltaic cells and electrolytic cells, based on the type of reaction occurring at the electrode.
Guidance
Tools and links
The nature of electricity
Direct current (d.c.) electricity is a flow of electrons from a region of negative potential to a region of positive potential. The negative electrons are attracted by the positive charge. This force of attraction is called the electromotive force (E.M.F.), and is measured as a voltage. It is also called the potential difference between the negative and positive terminals, or 'ends' of the voltage source.
The amount of current that can flow depends on how much 'push' or energy it's given by the voltage, as well as the resistance of the circuit. The relationship is a very simple one, called 'Ohms Law':
|
Voltage = Current x Resistance
V = IR |
The resistance of any given circuit is effectively constant, hence the current is directly proportional to the voltage applied.
| Note In physics the convention is to describe a flow of electrical current in the opposite direction to chemistry, i.e. from + to -. For this reason the physics version of current is often called 'conventional current', it flows from positive to negative. This arose historically because the laws of physics, as regards electricity, emerged before the discovery of the electron. In chemistry we know that electrons actually make up the current, so we've got it right! |
Electrical cells
As we have seen, certain species lose electrons (reducing agents) and other species gain electrons (oxidising agents) when reacting. If these species are not mixed together, but connected electrically by means of an external circuit, then these electrons will flow around the external circuit producing an electric current.
Each of the reacting species is then called a half cell and the whole set up is called an electrochemical cell. It is the basis behind the electrical battery.
In this cell the zinc metal has a tendency to dissolve as ions, leaving its electrons on the electrode. The copper, which is a weaker reducing agent, is forced to accept the electrons and use them to turn the copper ions into copper at the copper electrode. These electrons flowing around the outer (external) circuit constitute an electric current.
The 'salt bridge' is usually a filter paper soaked in potassium nitrate solution (neither of these ions react with any other ions in the experiment). This 'salt bridge' then allows ions to move in both directions, equalising any build up of electrical charge in the beakers.
Reactions in cells
The zinc forces the copper ions to accept electrons and the overall cell equation can be constructed by adding together the two 'half-equations' above.
|
Zn(s) ⇋
Zn2+(aq) + 2e
|
|
Cu2+(aq) + 2e ⇋
Cu(s)
|
|
overall: Zn(s) + Cu2+(aq) ⇋
Zn2+(aq) + Cu(s)
|
This type of cell can be constructed using any pair of reducing and oxidising agents. The greater the difference in the reactivity of one type of species (i.e. the reducing species) the greater the cell potential (voltage)
Consequently a cell constructed from zinc ¦ zinc sulfate in one half cell and silver ¦ silver nitrate solution in the other half cell will have a greater voltage that the cell above (there is a greater difference in reactivity between zinc and silver than between zinc and copper)
The negative electrode - anode
This is the electrode that actually produces the electrons that would flow around the external circuit. It is always the electrode of the most reactive metal (SL), the half cell with the most negative electrode potential (HL only).
The metal ions in the electrode dissolve as ions, leaving their electrons behind on the electrode. These electrons are then able to flow around the external circuit. For example in the zinc-copper voltaic cell the zinc half cell is the negative electrode: In voltaic cells this electrode is called the anode.
|
Zn(s) → Zn2+(aq) + 2e
|
The reactions at the negative electrode always involve electrons being 'dropped off' by metals in the electrodes dissolving as ions. This is a process of oxidation; the metal atoms are getting oxidised to ions (and releasing electrons).
The positive electrode
This is electrode towards which the electrons in the external circuit flow. It is the electrode that has positive ions (from the solution) removing the electrons as they arrive. In the case of the zinc-copper voltaic cell, the copper half cell is the positive electrode:
|
Cu2+(aq) + 2e → Cu(s)
|
In voltaic cells this electrode is called the cathode. It is the electrode at which the electrons are 'picked up' by the ions from the solution. The reactions occurring at this electrode are always reductions. The ions from the solution collect electrons to become atoms.
The cell reaction
The overall cell reaction can be obtained by adding together the reactions occurring at the positive and negative electrodes. If the number of electrons involved at both electrodes is different then the equation must be manipulated by mutiplication to make the number of electrons involved in each the same.
|
Example 1: The copper-zinc voltaic cell
In this case the number of electrons involved at both electrodes is the same, so the equations can be added together: Zn(s) → Zn2+(aq) + 2e Cu2+(aq) + 2e → Cu(s) Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) |
|
Example 2: The copper-silver voltaic cell
In this case the number of electrons involved at both electrodes is not the same, so before the the equations can be added together the silver equation must be doubled (multiplied by 2): Cu(s) → Cu2+(aq) + 2e 2Ag+(aq) + 2e → 2Ag(s) Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s) |
Worked examples
Q842-01 A voltaic cell is made from magnesium and iron half cells. Magnesium is a more reactive metal than iron. Which statement is correct when the cell produces electricity?- Electrons are lost from magnesium atoms
- The concentration of the Fe2+ ions increases
- Electrons flow from the iron half-cell to the magnesium half-cell
- Negative ions flow through the salt bridge from the magnesium half-cell
to the iron half-cell
|
As magnesium is the most reactive metal it preferentially becomes ions according to the equation:
The electrons produced flow around the external circuit to the iron half cell. Statement A is correct. |
Q842-02 A voltaic cell made from copper and zinc half cells has the equation shown below:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Which statement is correct when the cell produces electricity?
- Electrons are lost from zinc atoms
- The mass of the copper electrode decreases
- Electrons flow from the copper half-cell to the zinc half-cell
- Negative ions flow through the salt bridge from the zinc half-cell to the
copper half-cell
|
The equation shows us that the zinc provides electrons for the copper (II) ions, therefore statement A, electrons are lost from zinc atoms, is correct. |
Q842-03 What occurs during the operation of a voltaic cell based on the following reaction?
Ni(s) + Pb2+(aq) → Ni2+(aq) + Pb(s)
| External circuit | Ion movement in solution | |
|---|---|---|
|
A.
|
Electrons move from Ni to Pb | Pb2+(aq) moves away from Pb(s) |
|
B.
|
Electrons move from Ni to Pb | Pb2+(aq) moves towards Pb(s) |
|
C.
|
Electrons move from Pb to Ni | Ni2+(aq) moves away from Ni(s) |
|
D.
|
Electrons move from Pb to Ni | Ni2+(aq) moves towards Ni(s) |
|
The cell equation shows us that nickel atoms lose electrons and pass them around the external circuit to the lead ions. In the solutions the lead ions in the lead half cell pick up the electrons to become lead atoms. For this to happen, the Pb2+(aq) ions must move towards the Pb(s) electrode. The correct combination is selection B. |
Q842-04 In a voltaic cell, oxidation occurs at which of the following?
- anode
- cathode
- salt bridge
- electrode at which electrons enter from the outside
|
Oxidation is the process of electron removal (OILRIG = oxidation is loss, reduction is gain). The process of oxidation happens at the most reactive metal half-cell electrode: M(s) → M2+(aq) + 2e These electrons then flow arond the external circuit to the other electrode where they become available for reaction. This electrode, where the electrons are available is called the cathode. It is the positive side of the electro chemical cell. Hence oxidation occurs at the other electrode - the anode. |
Q842-05 Mercury batteries, like those used in electric watches, provide a voltage of 1.35 V. If the overall oxidation-reduction equation taking place is:
Zn(s) + HgO(s) + 2H2O(l) → Zn(OH)2(s) + Hg(l)
the anode reaction must be which of the following?
- HgO(s) + 2H2O(l) + 2e → Hg(l) + 2OH-(aq)
- Zn(s) + 2OH-(aq) → Zn(OH)2(s) + 2e
- Hg(l) + 2OH-(aq) → HgO(s) + 2H20(l) + 2e
- Zn(OH)2(s) + 2e → Zn(s) + 2OH-(aq)
|
The equation shows us that the zinc is turning from zinc atoms to zinc ions, i.e. its oxidation state is increasing from 0 to II. It is being oxidised. The Zn atoms are becoming Zn(OH)2(s) so the half-equation at the anode is:
The anode is the electrode where the electrons are produced by the metal and the metal is oxidised, it is the negative electrode, which in this case is the zinc. |
Q842-06 A battery consists of which type of cells?
- electrolytic
- electrochemical
- electroplating
- electromagnetic
|
Batteries make electricity from chemical reactions, so the correct answer is electrochemical |
Q842-07 Given the lead-acid battery reaction:
Discharge
Pb + PbO2 + H2SO4 → 2PbSO4 + 2H2O
Charge
2PbSO4 + 2H2O → Pb + PbO2 + H2SO4
Which species is oxidized during battery discharge?
- Pb
- PbO2
- SO42-
- H2O
|
When the battery discharges it is producing electricity. Therefore it is an electrochemical cell. The forward direction is when the cell discharges, thus the Pb is becoming PbSO4. However, lead is present in two forms, Pb and PbO2. This means that there are two equations involving lead, one is oxidation and the other is reduction:
In the first equation the lead is oxidised. |
Q842-08 Given the reaction for the nickel-cadmium battery:
2NiO(OH) + Cd +2H2O → 2Ni(OH)2 + Cd(OH)2
Which of the following species is oxidised during the discharge of the battery?
- Ni3+
- Ni2+
- Cd
- Cd2+
|
From the equation nickel changes from NiO(OH) in which it has an oxidation state of (III) to Ni(OH)2 in which it has an oxidation state of (II). The nickel has been reduced. Cadmium changes from the element to cadmium (II) - the cadmium has been oxidised. |
Q842-09 Which statement best describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell?
- It prevents the migration of electrons.
- It permits the migration of ions.
- It permits the two solutions to mix completely.
- It prevents the reaction from occurring spontaneously.
|
The salt bridge allows ions to flow in either direction to prevent any build up of charge in the half cells. The correct response is that it permits the migration of ions. |
Q842-10 The apparatus shown may be used to carry out a redox reaction.
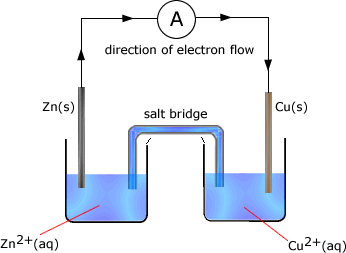
State the function of the salt bridge. [1]
Write the half-equation for the oxidation reaction. [1]
The above reaction are carried out under standard conditions. State what the
standard conditions are for the cell. [2]
Using the data
given calculate the cell potential. [2]
- Eº of Zn2+(aq)|Zn(s) = -0.76 V
- Eº of Cu2+(aq)|Cu(s) = +0.34 V
State and explain what happens to the concentration of copper(II) ions when
the cell is producing an electrical current. [2]
State two observations that could be made if the zinc rod were to be placed
in the solution of copper(II) ions. [2]
|
The salt bridge completes the circuit by allowing ions to flow in both directions to equalise charge in the two half cells. The oxidation reaction is:
Standard conditions are a temperature of 25ºC and 1.0 mol dm-3 solution concentrations. As the cell produces current, the copper ions in solution pick up electrons from the cathode and become copper atoms. The concentration of copper (II) ions decreases. If the zinc rod were placed in the copper(II) sulfate solution it would start to dissolve, the copper would be directly deposited on the surface of the zinc and the solution's blue colour would fade. |
