Standard level
Covalent compounds are held together by shared pairs of electrons. When the bond breaks the electrons may both go to the same atom, or one electron goes to each atom. Breaking a covalent bond is called bond fission.
Syllabus ref: R3.3.2Reactivity 3.3.2 - Radicals are produced by homolytic fission, e.g. of halogens, in the presence of ultraviolet (UV) light or heat.
- Explain, including with equations, the homolytic fission of halogens, known as the initiation step in a chain reaction.
Guidance
- The use of a single-barbed arrow (fish-hook) to show the movement of a single electron should be covered.
Tools and links
- Reactivity 1.2 - Why do chlorofluorocarbons (CFCs) in the atmosphere break down to release chlorine radicals but typically not fluorine radicals?
- Structure 2.2 - What is the reverse process of homolytic fission?
- Structure 2.2 - Chlorine radicals released from CFCs are able to break down ozone, O3, but not oxygen, O2, in the stratosphere. What does this suggest about the relative strengths of bonds in the two allotropes?
Heterolytic fission
Both of the electrons in the bond go with only one of the atoms. This results in the formation of charged particles - ions.
Saturated hydrocarbons cannot undergo this type of bond fission as the electron pairs are evenly shared between the carbon and hydrogen atoms.
Heterolytic fission is important in organic compounds with electronegative functional groups, such as haloalkanes.
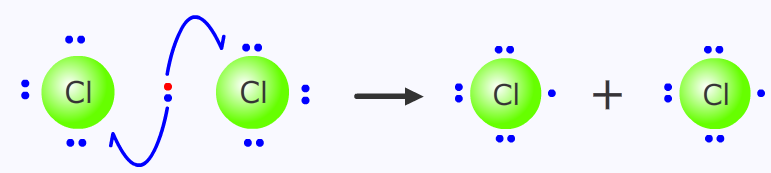
Homolytic fission
Homolytic fission involves bond cleavage where one electron goes off with each of the bonded atoms. This can occur with an input of energy, either thermal or electromagnetic, depending on the strength of the bond, or collision with free-radical initiators.
The chlorine-chlorine single bond is relatively weak, 242 kJ mol-1 making it susceptible to homolytic cleavage in the presence of electromagnetic radiation in the UV region of the spectrum.
Free-radical initiators
These are substances that have a very weak bond that breaks homolytically and reversibly making free-radicals. These free-radicals can then collide with the reagent molecule, creating a free-radical from the reagent. The reaction is thus initiated.
Organic peroxides, containing the O-O bond, and azo compounds, containing the N-N single bond, are used as free-radical initiators.
Representing electron movement
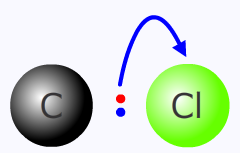
Heterolytic fission
By convention the movement of an electron pair during a reaction mechanism is shown using a curved arrow, called a "curly arrow". The arrow starts at the original location of the electron pair and ends at the destination.
heterolytic fission of the carbon - chlorine bond
Homolytic fission
By convention the movement of a single electron during a reaction mechanism is shown using a curved half-headed arrow, called a "fish-hook". The arrow starts at the original location of the electron and ends at the destination.
homolytic fission of chlorine