Standard level
An electrophile is a reactant that forms a bond to its reaction partner (the nucleophile) by accepting both bonding electrons from that reaction partner.
Syllabus ref: R3.4.4Reactivity 3.4.4 - An electrophile is a reactant that forms a bond to its reaction partner (the nucleophile) by accepting both bonding electrons from that reaction partner.
- Recognize electrophiles in chemical reactions.
Guidance
- Both neutral and positively-charged species should be included.
Tools and links
Electrophiles
Literally, species seeking electrons.
This means that an electrophile is either a positively charged species, a partially positive species, or is electron deficient, i.e. does not have a full outer shell of electrons.
Electrophilic behaviour can also be induced during the process of a collision, in which charge clouds (electrons) repel causing exposure of the positive nucleus that can then attract electrons pairs.
Positively-charged species
Positively charged species are cations. These may be formed in the process of a reaction, such as the nitronium ion, NO2+. In the reaction between aromatic compounds and nitrating agents, the nitrating mixture, consisting of sulfuric and nitric acid react together to form the nitronium ion.
H2SO4 + HNO3 → HSO4- + H2O + NO2+
The nitronium ion can then behave as an electrophile in the electrophilic substitution of benzene.
NO2+ + C6H6 → C6H5NO2 + H+
Electron deficient species
Certain molecules, such as aluminium chloride do not have a full outer shell of electrons, making them electron deficient. This allows them to easily accept an electron pair from another species. They are electrophiles - Lewis acids.
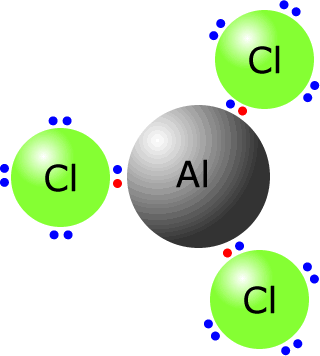
Partially positively-charged species
Effectively, this refers to polarised species that contain a partial positive "end". This can also be achieved by using molecules that polarise other molecules, such as Friedel Crafts catalysts.
Aluminium chloride is an electron deficient species that behaves as a Lewis acid catalyst, accepting electron pairs from other species forming polarised molecules that are able to act as Lewis acids themselves. The aluminium atom can accept an electron pair from a chlorine molecule, effectively polarising the molecule leaving a partially positive chlorine atom, that can then act as a Lewis acid.
AlCl3 + Cl2 → AlCl4----Clδ+
These reactions were studied by Friedel and Crafts and are very useful in the field of organic synthesis.
R-Cl + C6H6 → C6H5-R + HCl