Standard level
It has long been established that all matter is made up of particles. The world that we see and experience - the macroscopic world - is a direct consequence of the microscopic world of particles that we cannot see. The bulk (large scale) properties of matter that we are so familiar with, are a direct result of the interactions between the tiny particles that make up matter on a microscopic scale.
Syllabus ref: S1.1.3Structure 1.1.3 - The temperature, T, in Kelvin (K) is a measure of average kinetic energy Ek of particles.
- Interpret observable changes in physical properties and temperature during changes of state.
- Convert between values in the Celsius and Kelvin scales.
Guidance
- The kelvin (K) is the SI unit of temperature and has the same incremental value as the Celsius degree (°C).
Tools and links
- Reactivity 2.2 - What is the graphical distribution of kinetic energy values of particles in a sample at a fixed temperature?
- Reactivity 2.2 - What must happen to particles for a chemical reaction to occur?
The energy concept
It is an observation that when substances are heated their temperature increases. Although this may seem like an obvious statement, it is important to understand the processes that are involved. To do this we have to invoke the concepts of energy and temperature.
Energy is a concept that is very difficult to define without using examples. Certain forms of energy are easy to understand due to common experience, such as kinetic energy.
We appreciate that a moving body possesses 'kinetic energy' and that if something gets in its way then there will be collision and an effect (breakage, noise, deformation etc).
As the kinetic theory tells us that all particles are in motion, then we can refer kinetic energy to this motion and say that the particles have energy due to their movement. The total energy is the product of the average kinetic energy of a particle multiplied by the number of particles.
However, particles are not only in translational motion, they are also capable of vibrations and rotations. The total amount of motional energy they possess is a combination of these.
When a substance is given energy in the form of heat, the particles of the material respond by increasing their kinetic energy. The vibrations, rotations and translations of the particles in the material increase. We say that the substance has become 'hotter', although in fact all that has happened is that the particles are now moving faster.
In order to quantify this increase in particular kinetic energy, we must have a means of measuring and from here arises the idea of temperature scales and thermometers.
Temperature
Temperature is a measure the average heat energy content of the system under study.
The concept of 'heat' is easy to understand, as we have senses capable of detecting changes in heat. We say that something is warm or cold. However, the sensation of heat is just that, a sensation, something interpreted by the brain and understood by experience. What is actually occurring is a transfer of vibrations from the heat source to the sensors in the skin. These sensors produce an electrical signal that travels through nerve fibres to the brain, where we experience the sensation.
Our idea of 'heat' is simply the degree of vibrations experienced by the sensory cells. Clearly, this is OK for biological necessities, but hardly empirical. Scientists soon realised the need of enumerating this hot/cold reflex, and invented the thermometer.
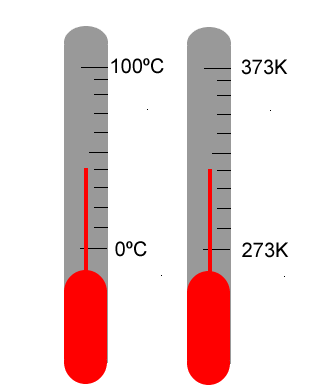
Recognising that certain materials respond to heat/cold by expansion/contraction, it was possible to enclose a sample of a suitable material into a glass tube and to watch it expand or contract as the local environment temperature changed. A scale was needed that was common experience for people all over the world. The two key reference points on the scale chosen were the freezing point (melting point) and boiling point of water. A scale was constructed between these two points and subdivided into 100 units (the Celsius scale). Nowadays the Celsius scale is the scale of choice for most purposes.
The thermometer responds to the average motion of the particles in the environment being measured. It should be recognised that when the thermometer registers 80ºC, it is informing us that the motion of the particles in the substance being measured has caused motion of the particles in the mercury, resulting in the mercury in the thermometer expanding to the 80º mark on the Celsius scale.
The Celsius scale is a relative scale, relative to the melting and boiling points of water. A more scientific scale is the absolute scale of temperature measurement in which the magnitude of a degree is the same as 1 degree Celsius, but the zero of the scale starts at absolute zero. This is also called the Kelvin scale; the unit of measurement is the Kelvin, K.
|
273 Kelvin = 0º Celsius 373 Kelvin = 100º Celsius |
Absolute zero is the temperature at which there is no particle motion whatsoever. It is equal to -273.16 ºC under current definitions.
Heat energy
The total energy of a sample is a function of both the temperature and the mass of particles present. The temperature gives a measure of the average kinetic energy of the particles in the system.
Example: Which contains the most energy, a bathful of lukewarm water or a spark at 2000ºC?
A spark may have a temperature of 2000ºC, but it contains less energy than a bathful of lukewarm water.
In reality, it is difficult to measure the absolute quantity of kinetic energy contained within a sample and it is of limited value. We are more concerned with changes in the heat energy, as this also reflects changes in the chemical energy of the system, as we shall see in the next section.
Remember! All substances at the same temperature have the same average kinetic energy.
The three states of matter
There are three states of matter, solids, liquids and gases. The state of a substance depends on the forces that hold its particles together and their average energy.
We can differentiate the bulk properties of the three states of matter in terms of shape and the volume they occupy:
| State | Solids | Liquids | Gases |
|---|---|---|---|
| Volume and shape | These have a fixed shape and a fixed volume | Have a fixed volume but no fixed shape | Have neither fixed shape nor volume |
These macroscopic properties are a direct result of the forces acting between the microscopic particles and the distances that these particles have between them.
Solids
The particles are close together and vibrate about fixed positions.
They cannot move any closer together and so the solid is incompressible.
The particles are held in fixed positions (apart from vibrations), which means that the solid keeps its shape in the macroscopic world unless subject to forces that bend, flatten or otherwise distort the shape.
Solids themselves can vary in properties, some being brittle while others are malleable, some hard while others are soft. These differences can always be explained by studying the underlying structure of the solid and the particles that comprise it.
Liquids
The particles of liquids are close together, but they are not in fixed positions. Their vibrations are enough to shake the whole structure apart and the particles can move with respect to one another.
The result of this freedom of motion is that the liquid flows in the macroscopic world and very small force is required to distort its shape. Gravity is enough to pull the liquid towards the earth and a liquid adopts the shape of the bottom of any container.
Where gravity doesn't act, for example in space, the liquid adopts a spherical shape. This indicates that forces act between the particles pulling them together.
This interparticular force is also responsible for the surface tension of liquids.
Gases
The energy of the particles makes them move rapidly and prevents any appreciable forces of attraction acting between the particles. The spaces between the particles are now very large and the gas is compressible; the particles can be easily pushed closer together.
The particles spread out, where possible, by constant collisions and expand to fill the entire space available.
Gases adopt the shape of a container and exert a pressure on the walls of the container.
Examples of molecular gases
- Hydrogen, H2
- Oxygen, O2
- Chlorine, Cl2
- Carbon dioxide, CO2
- Ammonia, NH3
Changes of state
Melting point (mp)
If the vibrational energy of the particles is less than the force holding them together, the substance is a solid.
As the temperature increases so the vibrations increase until eventually the structure breaks apart. We call this temperature the melting point of the solid.
If this temperature is approached from above and a liquid gradually cooled, then this is also the temperature at which the liquid solidifies or freezes.
Thus, the definition of melting point is the temperature at which solid and liquid states are in equilibrium (i.e. co-exist). For example, water is a solid below 0ºC and a liquid above 0ºC. At the melting point of ice, both water and ice co-exist in equilibrium with one another.
ice ⇋ water
Example: In what state is nitrogen at 56K, if its melting point and boiling points are 63K and 76K respectively?
56K is below the melting point and therefore the nitrogen is in the solid state at this temperature. The temperature would have to be increased to 63K in order for it to liquefy.
Boiling point (bp)
The same argument cannot be applied to the boiling point, as there are always particles in the body of a liquid that have sufficient energy to escape the forces of attraction within the liquid and escape into the gaseous state. This happens because the energy of the particles is distributed statistically, with some receiving a lot of energy whereas others receive very little.
This distribution of energy is shown by the Maxwell-Boltzmann energy distribution
The curve is temperature dependent and the average energy of the particles is proportional to the absolute temperature.
In the curve you can see that there are always some particles with high enough energy to overcome the forces of attraction and escape from the rest.
If the particles escaping from a liquid are carried away by currents of air the liquid evaporates.
Vapours
We call a gas that exists below the normal boiling point of a liquid a 'vapour'. The particles of vapour exert a pressure in the same way as any other gas; this is called the vapour pressure.
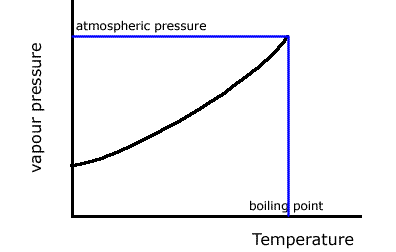
The boiling point of a liquid is the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure and bubbles of gas can spontaneously form within the liquid. This clearly depends on the atmospheric pressure at the time.
At the boiling point (or very slightly above it) the gas particles have too much energy to allow forces to pull them back together again.
The graph shows how the vapour pressure of a liquid steadily increases until it reaches atmospheric pressure. At this temperature the liquid boils. We record the boling point and the atmospheric pressure at the time.
Boiling points are useful measures of purity of liquids, as impurities cause a increase in the bp. This is called a colligative property.
Summary
External video resources
Temperature and particular kinetic energy: Richard Thornley
