Standard level
Hydrogen has one of the simplest emission spectra as there is only one electron per atom that can absorb and re-emit energy.
Syllabus ref: S1.3.2Structure 1.3.2 - The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
- Describe the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.
Guidance
- The names of the different series in the hydrogen emission spectrum will not be assessed.
Tools and links
- Inquiry 2 - In the study of emission spectra from gaseous elements and of light, what qualitative and quantitative data can be collected from instruments such as gas discharge tubes and prisms?
- Nature of science, Structure 1.2 - How do emission spectra provide evidence for the existence of different elements?

The Hydrogen emission series
The hydrogen emission spectrum

The electron in the ground state energy level of the hydrogen atom receives energy in the form of heat or electricity and is promoted to a higher energy level.
It cannot remain at a higher level (excited state) for very long, and falls back to a lower level.
When the electron falls back down (relax) it must lose the energy difference between the two energy levels. This loss of energy is performed by releasing electromagnetic energy in the form of infrared, visible light or ultraviolet radiation.
Movement of electrons between the shells is called electron transitions.
When electron transitions take place the energy emitted can be detected and its wavelength measured. This provides information about the relative energies of the shells.
In the hydrogen atom (the simplest case with only one electron to 'jump' between shells) the energy emitted appears in several series of lines, each series corresponding to electrons falling back to different levels. This is shown in the diagram below.
The Lyman series corresponds to transitions between the higher shells and the lowest shell (ground state). The energy of these transitions produces radiation in the ultra-violet region of the spectrum
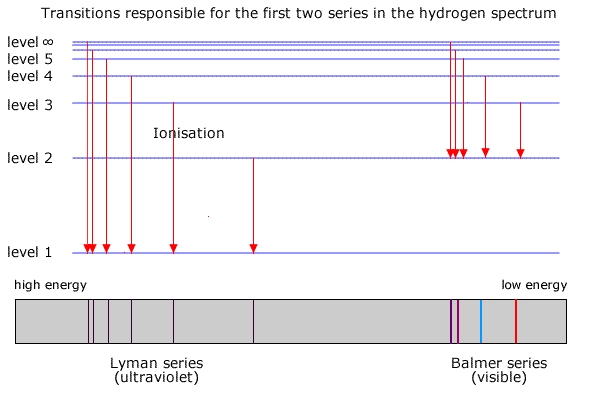
The energy shells are usually given a letter 'n' to describe the specific energy level. The lowest level is n=1, the second level is n=2 etc.
Transitions from higher shells (n > 2) to n = 2 emit radiation in the visible region of the spectrum. It can be seen by splitting the light using a prism or diffraction grating and projecting it onto a screen.
Worked examples
Q133-01 The lines in the series that appears in the visible region of the hydrogen spectrum are caused by transitions to which energy level?Answer
|
The series are, in order of decreasing energy:
|
Q133-02 Which phrase correctly completes the sentence. The lines in a series in the hydrogen spectrum...
- Converge towards the high energy side
- Diverge towards the high energy side
- Are evenly spaced
- Converge towards the IR region
|
The easiest way to remember this is to think about the ionization as the highest energy transition, from n=1 to n=∞. Convergence occurs towards this transition and so it is towards the highest energy end of each series. Correct response - A |
Q133-03 An electron can move from one orbital of a hydrogen atom to another. In which movement will the photon of highest energy be emitted?
- 2p ---> 1s
- 1s ---> 2p
- 3s ---> 2s
- 2s ---> 3s
|
The largest transitions occur in the ultraviolet series down to n=1 (the ground state). Correct response - A |
Q133-04 In which electronic state would a hydrogen atom be able to absorb a photon of electromagnetic radiation but not be able to emit a photon? (HL only)
- 1s1
- 2s1
- 3p1
- 4d1
|
Photons of energy cannot be emitted from the ground state as there is no lower state for the electron to go to . 1s1 represents the lowest orbital of hydrogen therefore this is the correct response. Correct response - A |
Q133-05 The emission spectrum of the hydrogen atom
- is caused by the removal (ionization) of the electron.
- is continuous because the electron can emit any frequency of light during a transition.
- is caused by the absorption of light at characteristic frequencies the electron to be excited into higher energy levels.
- is a result of the excited electron undergoing transitions to lower energy levels and emitting photons of light at specific frequencies.
|
Transitions of electrons to lower levels emit the extra energy in the form of electromagnetic radiation. Correct response - D |
Q133-06 Which transition is associated with the largest change in energy in the hydrogen atom?
- n = 5 to n = 3
- n = 2 to n = 1
- n = 3 to n = 2
- n = 4 to n = 2
|
The largest energy transitions are down to the n=1 level. Correct response - B |
Q133-07 According to the evidence supplied by the hydrogen spectrum, which transition involves the greatest energy difference, n=2 to n=1 or n=∞ to n=2?
Answer
|
The Lyman series (transitions to n=1) and the Balmer series (transitions to n=2) do not overlap. This means that the energy involved in each one of the transitions down to n=1 is greater than any of the transitions to n=2. The greatest energy difference wil be due to the transition n=2 to n=1 |
Q133-08 What name is given to an electron that is in a higher energy level than it should be?
- transition electron
- excited electron
- relaxed electron
- ground state electron
|
The term 'excited' is used to indicate that the electron has more energy than it should have. It is in an excited state. |
Q133-09 Which transition corresponds to the ionization energy of the hydrogen atom?
|
For ionization to occur the electron must leave the (gaseous) atom. M(g) → M(g)+ + 1e The ionization energy is always measured from the ground state, so the transition is from n = 1 to n = infinity. This is expressed as: n = 0 → n = ∞ |
Q133-10 The term 'relaxation' refers to which of the following in the context of the hydrogen spectrum:
- The dark gap between the different series of the hydrogen spectrum.
- The difference in energy between the lines in the Balmer and Lyman series
- The movement of an electron from a higher to a lower energy level
- The movement of an electron from a lower to a higher energy level
|
To relax, in the context of spectra, means to move from a higher energy level to a lower energy level. |
External video resources
The Line Spectrum of Hydrogen: Richard Thornley
