Higher-level only
Atoms are too small to be seen. We can only obtain information indirectly by stimulating the atoms with energy of various forms and detecting what emerges.
Syllabus ref: S1.3.7Structure 1.3.7 - Successive ionization energy (IE) data for an element give information about its electron configuration. (HL)
- Deduce the group of an element from its successive ionization data.
Guidance
- Databases are useful for compiling graphs of trends in IEs.
Tools and links
- AHL Structure 3.1 - How do patterns of successive IEs of transition elements help to explain the variable oxidation states of these elements?

Successive ionization energies
The ionization energy of the elements can be determined by several means. These are beyond the scope of the Syllabus. Students must understand the definitions of 1st and successive ionization energies and also the factors that affect them, specifically electrostatic forces.
The first ionization energy
The first ionization energy is defined as the energy required to remove one mole of electrons from one mole of gaseous atoms to provide one mole of gaseous single charged ions.
Na(g) → Na+(g) + 1e
Subsequent ionization energies are defined in a similar way only by removing electrons from already charged ions.
The second ionization energy
Na+(g) → Na2+(g) + 1e
Successive electrons can be stripped from an atom until there is only the nucleus left. If the energy required to achieve this for each ionization is plotted on a graph (with a log scale) against the ionization number, the 'jumps' in the required energy clearly show the main and sub energy levels.
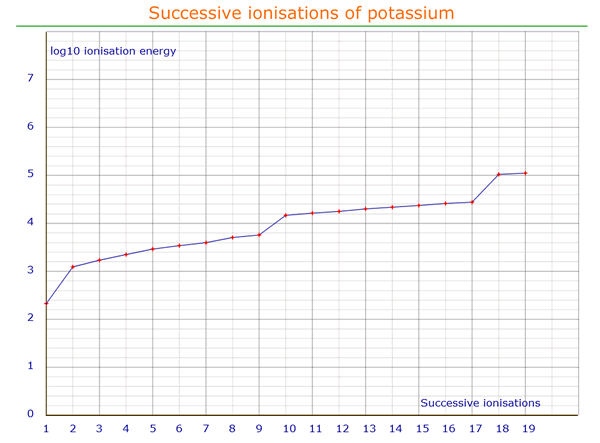
In this example, it may be seen that removal of the first electron requires (relatively) less energy than removal of the next (eight) electrons - there is a distinct inflexion (change of direction) in the otherwise fairly linear graph. Consequently the element concerned must be in group I.
Many exam questions focus on the ability of a student to recognise this inflexion from purely numerical data and then ask for details of its group in the periodic table.
Example: In the following table identify the groups to which the elements X, Y and Z belong (all values in kJ mol-1).
| element | 1st I.E. | 2nd I.E. | 3rd I.E | 4th I.E. |
| X | 496 | 4562 | 6912 | 9543 |
| Y | 738 | 1451 | 7732 | 10540 |
| Z | 578 | 1817 | 2745 | 11577 |
It may be seen that the inflection (relatively bigger jump) for element X occurs between 1st and 2nd ionization energies. It is therefore in group 1. Similarly the inflection for Y occurs between the 2nd and 3rd ionization energies and so it is in group 2.
Successive ionization energy of an element
Worked examples
Q136-01 The 1st ionization energy of boron is slightly less than that of beryllium. This is best explained by which of the following?- The electron lost from the boron atom is in a 'p' orbital
- The electron lost from the boron atom is repelled by other electrons
- The electron lost from a boron atom is only attracted by five protons in the nucleus
- The electron lost from a boron atom is highly energetic
Answer
|
The electronic configuration of boron is 1s2 2s2 2p1 The electronic configuration of beryllium is 1s2 2s2 The 2p electron is further away from the nucleus and therefore easier to remove. Response A |
Q136-02 The first seven ionization energies of an element are 1010, 1900, 2900, 5000, 6300, 21 300 and 25 400 kJ/mole respectively. In which group of the Periodic Table is the element?
- 4
- 5
- 6
- 7
|
For these questions we look for an inflexion in the line (the point at which the slope changes dramatically). By examination of the data, we can see that there is a disproportionate jump between ionisations 5 and 6. This means that the sixth electron removed came from an orbital closer to the nucleus than the fifth. Hence there are five electrons in the outer shell, the element is from group 5. |
Q136-03 From which of the following can the value for the ionization energy of hydrogen be calculated:
- I. The value of Planck's constant in kJ mol-1 s
- II. The value of Avogadro's constant
- III. The frequency of the convergence limit of the lines in the ultraviolet emission spectrum of hydrogen
- I only
- I and II ionly
- I and III only
- I, II and III
|
The convergence limit in the U.V. hydrogen spectrum is the transition from n = 1 to n = ∞, i.e. the ionization frequency. The frequency is related to the energy of the wave by the equation E=hf Where h = Planck's constant This equation gives the energy for 1 transition therefore for 1 mole of transitions it must be multiplied by Avogadro's constant. The correct response is I, II and III are needed. |
Q136-04 The following graph of log ionization energy against ionization number is likely to be of an element in which group?
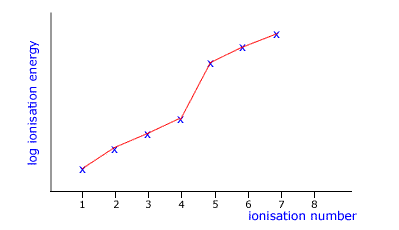
|
You can see a point of inflexion (change in direction) after the fourth ionisation. This suggests that the fifth element comes from a shell that is closer to the nucleus. For this to be the case the original element must be in group IV. |
Q136-05 The following diagram shows a section of the graph of 1st ionization energy against atomic number. In which group of the periodic table is the element labelled A?
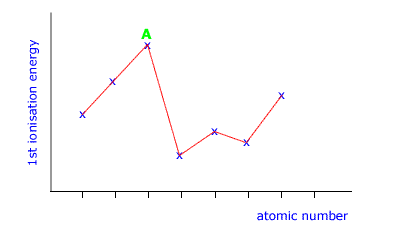
|
The peaks in this graph show those elements with the highest ionization energy. These are the inert gases, group 18 (also called group 0) |
Q136-06 In the graph of 1st ionization energy against atomic number, why is there a general trend towards higher ionization energy moving across a period?
Answer
|
The increased nuclear charge moving across a period has a progressively greater attraction for the outer electron within the same shell. |
Q136-07 On which two factors does the magnitude of the electrostatic attraction between opposite charges depend?
Answer
|
The magnitude of the attracting charges and the distance between them.
|
Q136-08 Why is the energy required to remove an electron from an oxygen atom less than that required to remove an electron from a nitrogen atom?
Answer
|
The nitrogen atom has a configuration of 1s2 2s2 2p3 whereas the oxygen atom has a configuration of 1s2 2s2 2p4. Even though the electron being removed is in the same energy shell, the oxygen has the electron paired in a 'p' orbital and suffers repulsion from its pairing electron. This means that less energy is required to remove. |
Q136-09 Why is the 1st ionization energy of potassium less than that of sodium?
|
Potassium has a larger positive nuclear charge, BUT the outer electron is in the fourth shell and much further from the nucleus. Potassium also has inner electron shells that contribute a repulsion effect, reducing the attraction of the outer electron for the nucleus. This is sometimes called 'shielding'. |
Q136-10 Why is the first ionization energy of the 1st row transition elements fairly constant?
|
The first electron lost by the transition elements is the 4s electron. These electrons are shielded by the inner 'd' orbitals as they get filled moving across the period. The effect of adding a proton to the nucleus and adding an inner 'd' electron more or less cancel each other out. This makes the force of attraction between the outer electron and the nucleus approximately constant moving across the period. Hence the 1st ionization energy is approximately constant. |