Standard level
Solutions are one of the commonest ways of performing reactions, as the particles of solute are free to move and collide. It is, however, important for the chemist to know how many particles there are per unit volume of a solution and for this the concept of concentration and molarity are used
Syllabus ref: S1.4.5Structure 1.4.5 - The molar concentration is determined by the amount of solute and the volume of solution.
- Solve problems involving the molar concentration, amount of solute and volume of solution.
Guidance
- The use of square brackets to represent molar concentration is required.
- Units of concentration should include g dm–3 and mol dm–3 and conversion between these.
- The relationship n = CV is given in the data booklet.
Tools and links
- Tool 1 - What are the considerations in the choice of glassware used in preparing a standard solution and a serial dilution?
- Tool 1, Inquiry 2 - How can a calibration curve be used to determine the concentration of a solution?
Concentration
The concentration of a solution is the quantity of solute that it contains per unit volume.
This may be given in grams per 100cm3 or grams per litre, but it is usually given in terms of molarity as this gives a direct measure of the number of solute particles contained by the solution.
Molarity
The concept of molarity arises from the need to know the amount of solute present in a solution in moles. 1 mole of any substance contains an Avogadro number of particles of that substance = 6.02 x 1023. A 1 molar solution contains 1 mole of solute, dissolved in 1 litre of solution.
Note: the definition is not per 1 litre of solvent, but per 1 litre of solution. This allows us to measure a volume of solution and work out the number of moles, and hence the number of particles, that it contains.
Molarity = number of moles of solute per litre of solution (1 litre = 1000cm3)
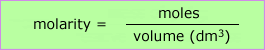
The molarity is denoted by the capital letter M, and given the units mol dm-3
|
Example: Calculate the molarity of a solution containing 0.15 moles of potassium nitrate in 100cm3 of solution. Molarity = moles/litres 100cm3 = 0.1dm3 Molarity = 0.15/0.1 = 1.5 M |
The molarity of a specific ion within an ionic solution may also be considered separately. In a 1M solution of copper sulfate (CuSO4) the copper ions are separate from the sulfate ions. The solution may be said to be both 1 molar in terms of copper 2+ ions and 1 molar in terms of sulfate 2- ions.
A 1 molar (1M) solution of copper nitrate (Cu(NO3)2), however, is 1 molar with respect to copper 2+ ions but 2M with respect to nitrate ions.
Example: Calculate the molarity of hydrogen ions in a 0.15 molar solution of sulfuric acid.
The formula of sulfuric acid is H2SO4. It dissociates in solution according to the following equation:
H2SO4 ⇋ 2H+ + SO42-
Hence, if a solution is 1 molar in sulfuric acid, it must be double that in hydrogen ions.
Molarity of the solution = 0.15M in sulfuric acid,
Therefore the molarity in hydrogen ions = 0.15 x 2 = 0.3 mol dm-3
Using:
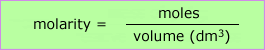
In conjunction with
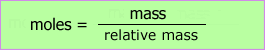
We can calculate the mass needed to prepare solutions or the mass contained in solutions of known concentration.
Example: Calculate the mass of iron(II) sulfate in 100 cm3 of 0.1 mol dm-3 solution.
The formula of sulfuric acid is FeSO4. It has a relative formula mass = 56 + 32 + 64 = 152
Number of moles in 100cm3 of 0.1 mol dm-3 solution = 0.1 x 0.1 = 0.01 moles
Therefore mass of iron /(II) sulfate = moles x relative formula mass = 0.01 x 152
Therefore mass of iron(II) sulfate = 1.52 g
The first of the above mathematical formulae can be manipulated by rearrangement to obtain any of the three factors, moles, molarity or volume of solution.
Molality
The molality of a solution is the mass of solute per kiologram of solvent. It has limited use.
Worked examples
Calculate the molarity of the following solutions.
Q351-01 3.65 g of HCl in 1000 cm3 of solution
Answer|
Mr of HCl = 35.5 + 1 = 36.5 3.65g = 3.65/36.5 moles = 0.1 moles 1000 cm3 = 1 litre Molarity of solution = moles/litres Molarity of solution = 0.1/ 1 = 0.1 M |
Q351-02 3.65 g of HCl in 100 cm3 of solution
Answer|
Mr of HCl = 35.5 + 1 = 36.5 3.65g = 3.65/36.5 moles = 0.1 moles 100 cm3 = 0.1 litre Molarity of solution = moles/litres Molarity of solution = 0.1/ 0.1 = 1.0 M |
Q351-03 1.00 g of NaOH in 250 cm3 of solution
Answer|
Mr of NaOH = 23 + 16 + 1= 40 1.00 g = 1.00/40 moles = 0.025 moles 250 cm3 = 0.25 litre Molarity of solution = moles/litres Molarity of solution = 0.025 / 0.25 = 0.1 M |
Q351-04 1.96 g of H2SO4 in 250 cm3 of solution
Answer|
Mr of H2SO4 = [2 x 1]+ 32 + [4 x 16] = 98 1.96 g = 1.96/98 moles = 0.02 moles 250 cm3 = 0.25 litre Molarity of solution = moles/litres Molarity of solution = 0.02 / 0.25 = 0.08 M |
Q351-05 25.0 g of Na2S2O3.5H2O in 250 cm3 of solution
Answer|
Mr of Na2S2O3.5H2O = [2 x 23]+ [2 x 32] + [3 x 16] + [5 x 18] = 248 24.8 g = 24.8/248 moles = 0.1 moles 250 cm3 = 0.25 litre Molarity of solution = moles/litres Molarity of solution = 0.1 / 0.25 = 0.4 M |
Q351-06 Calculate the concentration of hydrogen ions in a 2M solution of hydrochloric acid.
Answer|
Hydrochloric acid is a strong acid and completely dissociates into ions in aqueous solution: HCl → H+ + Cl-1 mole of HCl produces 1 mole of H+ ions Concentration of hydrogen ions is therefore the same as the concentration of hydrochloric acid = 2 moles dm-3 |
Q351-07 Calculate the concentration of hydrogen ions in a 2M solution of sulfuric acid.
Answer|
sulfuric acid is a strong dibasic acid and dissociates 100% in solution. H2SO4 → 2H+ + SO42-1 mole of sulfuric acid produces 2 moles of hydrogen ions The 2 M solution of sulfuric acid is therefore 2 x 2 = 4 M with respect to hydrogen ions |
Q351-08 Calculate the molarity of the sodium ions in solution when 10.6g of anhydrous sodium carbonate is dissolved in 50cm3 of water and the volume made up to 100cm3.
Answer|
Relative formula mass of sodium carbonate, Na2CO3 = 106 10.6g of sodium carbonate = 10.6/106 moles = 0.1 moles But when 1 mole of Na2CO3 dissolves in water it produces 2 moles of Na+ ions Therefore moles of sodium ions = 0.1 x 2 = 0.2 moles Total volume of solution = 100cm3 Molarity = moles/ volume(litres) = 0.2/0.1 = 2 M with respect to sodium ions |
Q351-09 Calculate the molarity of the chloride ion solution produced, when 1.345 g of copper chloride is dissolved in 100cm3 of water and the solution volume made up to 250cm3.
Answer|
Relative formula mass CuCl2 = 63.5 + 2(35.5) = 134.5 Moles of copper chloride = 1.35/134.5 = 0.01 moles Total volume of solution = 250cm3 Molarity of the copper chloride solution = moles/volume(litres) = 0.01/0.25 = 0.04 M But 1 mole of copper chloride produces 2 moles of chloride ions Therefore molarity with respect to chloride ions = 0.04 x 2 = 0.08 moles dm-3 |
Q351-10 Calculate the molarity of the potassium carbonate solution formed by absorbing 4.4g of carbon dioxide in 500cm3 of 2M potassium hydroxide solution, according to the equation:
2KOH + CO2 → K2CO3 + H2O
Answer|
Moles of carbon dioxide = 4.4/44 = 0.1 moles From the equation for the reaction, 1 mole of carbon dioxide produces 1 mole of potassium carbonate Therefore moles of potassium carbonate formed = 0.1 moles Total volume of solution = 500cm3 Thefore molarity of potassium carbonate = 0.1/0.5 = 0.2 moles dm-3 |
Now test yourself
External video resources
Solving solution problems: Richard Thornley