Standard level
'Poly' = many, therefore polyatomic = many atoms.
Syllabus ref: S2.1.3Structure 2.1.3 - Ionic compounds exist as three-dimensional lattice structures, represented by empirical formulas.
- Explain the physical properties of ionic compounds to include volatility, electrical conductivity and solubility.
Guidance
- Include lattice enthalpy as a measure of the strength of the ionic bond in different compounds, influenced by ion radius and charge.
Tools and links
- Tool 1, Inquiry 2 - What experimental data demonstrate the physical properties of ionic compounds?
- Structure 3.1 - How can lattice enthalpies and the bonding continuum explain the trend in melting points of metal chlorides across period 3?
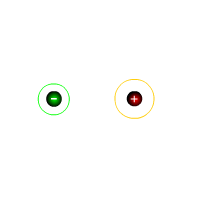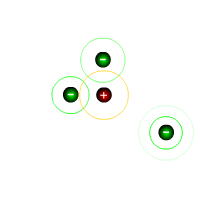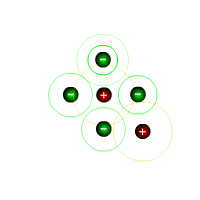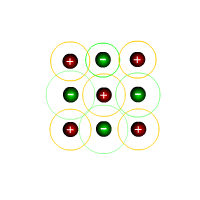
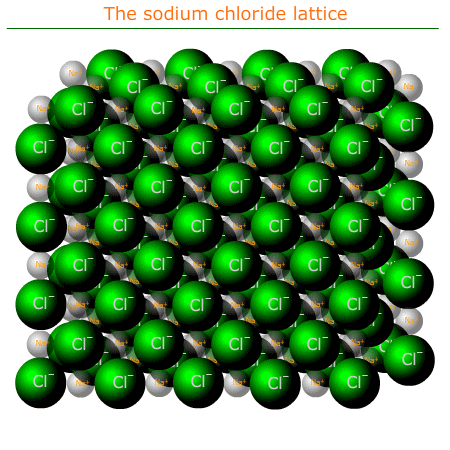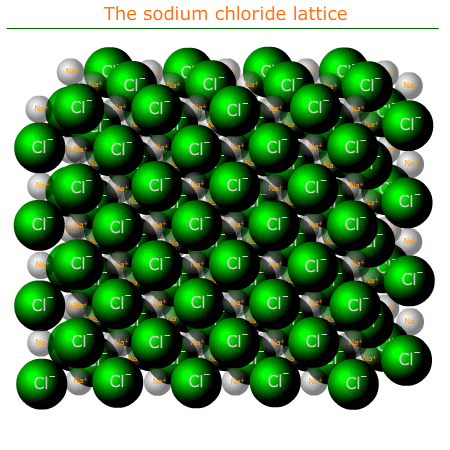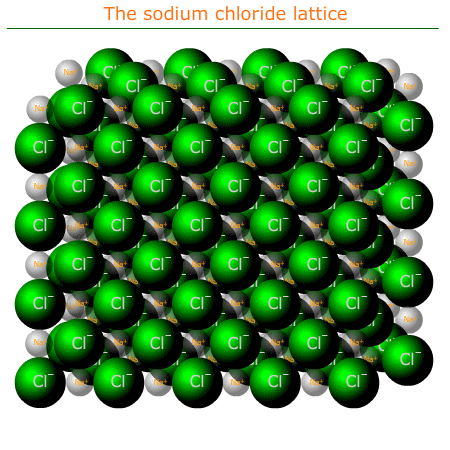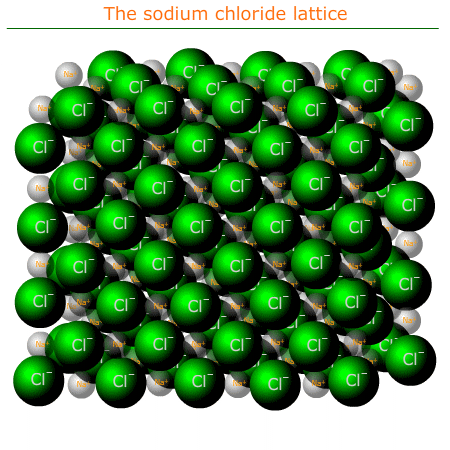
The ionic lattice
An ionic lattice is a regular repeating arrangement of ions in three dimensions. The ions ocupy files and rows and columns. Each negative ion is surrounded by positive ions and each positive ion is surrounded by negative ions. The actual packing structure of the ions depends on the relative sizes of the ions and their respective charges.
Lattice strength and energy
The lattice strength depends on the forces of attraction between the positive and negative ions. This electrostatic force depends on two factors:
- The magnitude of the charge on the ions
- The distance between the ions (the sum of the ionic radii)
The equation for determining the electrostatic force of attraction is: 
In this equation the term 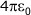 is just a series of constants and can be combined together to give:
is just a series of constants and can be combined together to give: 
In summary, the force of attraction between two ions is proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
The strength of a lattice is a function of its lattice enthalpy, the energy required to overcome the forces of attraction keeping the ions togerther in the lattice. This is defined as the energy required to separate one mole of a crystal lattice into gaseous ions at infinite separation. For example sodium chloride lattice enthalpy is given by:
NaCl(s) → Na+(g) + Cl-(g) ΔH = +790 kJ mol-1
Ions with a double charge produce lattices with a much higher lattice enthalpy.
|
Example: Compare the magnitude of electrostatic force in the lattices MgO and NaCl, given the following information:
In the MgO lattice both of the ions have a double charge so the product of the charges is for times as great as for NaCl. The sum of ionic radii for MgO = 0.205nm and for NaCl is 0.279nm. Squaring both terms we get: for MgO: 0.2052 = 0.0420, for NaCl: 0.2792 = 0.078. Therefore the r2 term for NaCl is aproximately twice as large as that of MgO. We would expect this to have the effect of doubling the electrostatic force. So the electrostatic force in the MgO lattice is has a numerator four times greater than NaCl and a denominator half as large as NaCl. Clearly the force of electrrostatic attraction in MgO is roughly eight times greater than in NaCl. Note: You will not be expected to do a mathematical treatment of ionic force in the exams, but it is useful to compare the forces involved. |
Sodium chloride structure
Sodium chloride, NaCl, can be considered a typical ionic compound. The structure often appears in examinations and should be familiar to students. It can be used as an example of the bonding and structure of all of the compounds formed between group 1 and group 17 elements.
Perfect and imperfect lattices
In some substances the calculated lattice energies vary considerably from the experimentally determined values. This is usually because the lattice has a degree of covalent character. What does this mean?
An ionic substance is made by electron transfer from metal atoms to non-metal atoms creating ions. In a perfect ionic substance these ions are not affected by one another. However, when the negative ion is large and the positive ion is small (or/and has a high charge), the high charge density of the positive ion can polarise the electron charge cloud of the large negative ion, pulling electrons back towards itself. This gives a distorted ionic charge field, or to put it another way, a degree of covalent character.

If the lattice has a degree of covalent character, it is tending towards simple molecular behaviour, in which the lattice is much weaker. The experimentally determined lattice energy is correspondingly smaller than the theoretically determined value.
Summary
All ionic compounds exist in a giant ionic lattice of repeating oppositely charged ions. The formula of the ionic compound is the simplest ratio between the ions within the lattice. For this reason the term "relative formula mass" is used when dealing with an ionic compound.
The strength of the ionic lattice is a function of both the charge on each ion and the radius of the ions.
- Greater charge = stronger lattice
- Smaller size = stronger lattice
This can be demonstrated by both the lattice enthalpy and the melting point of the lattice. The degree of ionic character is determined by the difference in electronegativity between the elements involved in the compound.
- Greater difference in electronegativity = more ionic character
Decreasing the degree of ionic character decreases the lattice strength and melting point.
Worked examples
Q264-01 In which of the following are the compounds CaF2, CaCl2, CsF and LiF arranged in order of increasing lattice enthalpy- CaCl2, CaF2, CsF, LiF
- CsF, LiF , CaCl2, CaF2
- CaCl2, CaF2, LiF, CsF
- LiF, CaF2, CsF, CaCl2
|
The two factors to be taken into consideration are the charges on the ions and the size of the ions. Calcium ions have a double positive charge and form the strongest electrostatic forces. The strongest lattices are those that contain calcium ions. Comparing calcium chloride and calcium fluoride, we have to look at the size of the halide ion. Fluoride ions are smaller than chloride ions and have a greater charge density. Thus the strongest lattice is calcium fluoride followed by calcium chloride. The other two compounds are caesium fluoride and lithium fluoride. In this case it is the size of the cation (positive ion) that changes. The smaller the ion the stronger the force, therefore lithium fluoride has a stronger lattice than caesium fluoride. The final order of increasing lattice enthalpy is: CsF, LiF , CaCl2, CaF2 |
Q264-02 Which statement is true for most ionic compounds?
- They contain elements of similar electronegativity?
- They conduct electricity in the solid state
- They are coloured
- They have high melting and boiling points
|
Ionic compounds all have high melting and boiling points. |