Standard level
Chromatography is a separation technique that depends on the intermolecular forces present in covalent compounds.
Syllabus ref: S2.2.10Structure 2.2.10 - Chromatography is a technique used to separate the components of a mixture based on their relative attractions involving intermolecular forces to mobile and stationary phases.
- Explain, calculate and interpret the retardation factor values, RF.
Guidance
- Knowledge of the use of locating agents in chromatography is not required.
- The technical and operational details of a gas chromatograph or high-performance liquid chromatograph will not be assessed.
Tools and links
- Tool 1 - How can a mixture be separated using paper chromatography or thin layer chromatography (TLC)?
Capillary action
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of external forces (like gravity). This phenomenon is most commonly observed when a liquid rises in a thin tube, known as a capillary tube. The forces of attraction between the particles of the liquid and the surrounding surfaces are key to understanding capillary action. These forces can be divided into two main types:
- cohesive forces
- adhesive forces
Cohesive Forces
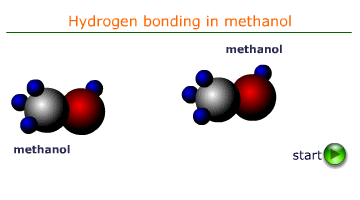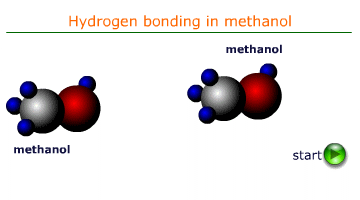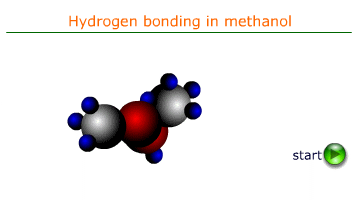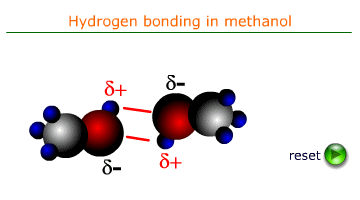
Cohesive forces are the intermolecular forces that attract molecules of the same substance to one another. In liquids, these forces are primarily due to hydrogen bonding, van der Waals forces, or dipole-dipole interactions. In water the most important of these forces is H-bonding. It is the force that is responsible for surface tension and the formation of a meniscus in a tube of water.
Adhesive Forces
Adhesive forces are the attractive forces between the molecules of a liquid and the molecules of a solid surface. These forces occur due to interactions such as hydrogen bonding, van der Waals forces, and other types of intermolecular attractions.
How Capillary Action Works
When a liquid comes into contact with a solid surface, adhesive forces cause the liquid molecules to be attracted to the surface. For example, water molecules are attracted to the glass in a capillary tube due to hydrogen bonding and polar interactions.
As the liquid spreads along the surface, cohesive forces pull more liquid molecules along with it, causing the liquid to rise in the tube. The balance between cohesive and adhesive forces determines how high the liquid will rise.
Example of capillary action - Water in a Glass Tube
Water rises in a thin glass tube due to the strong adhesive forces between the water molecules and the glass. The cohesive forces among water molecules help to pull additional water up the tube. The higher the column of water in the tube the greater the force of gravity pulling it back down.
Summary
Capillary action is a result of the balance between cohesive forces (attraction between like molecules) and adhesive forces (attraction between unlike molecules). The interplay of these forces allows a liquid to move through narrow spaces against external forces like gravity.
General chromatography
Chromatography is a technique used to separate and analyze the components of a mixture. It involves passing the mixture dissolved in a "mobile phase" through a "stationary phase," which separates the components based on their different interactions with these phases.
The mixture to be separated is introduced into the chromatographic system.
As the mobile phase moves through the stationary phase, different components of the mixture move at different rates. Components that interact more strongly with the stationary phase move more slowly, while those that interact less move faster.
How chromatography works
The separated components are detected as they exit the system (elute). Different methods such as UV-Vis spectroscopy, mass spectrometry, or flame ionization detectors can be used for detection.
Types of Chromatography:
- 1. Paper Chromatography
- 2. Thin Layer Chromatography (TLC)
- 3. Column Chromatography
- 4. Gas Chromatography (GC)
- 5. High-Performance Liquid Chromatography (HPLC)
Paper Chromatography:
Stationary Phase: Paper
Mobile Phase: Solvent (e.g., water or alcohol)
Application: Separation of pigments, inks, and small organic molecules
The retardation (retention) factor
Each solute has a different attraction to both the mobile phase and the stationary phase. This means that different solutes travel through the stationary phase at differing rates. The distance reached by a solute divided by the distance reached by the solvent front gives a fraction known as the retention (or retardation) factor, RF.
Separation of pigments
This is the most commonly used chromatography in schools. A typical experiment would separate the two different forms of chlorophyll (A & B) from a leaf extract.
2. Thin Layer Chromatography (TLC):
Stationary Phase: Thin layer of silica gel or alumina on a glass/plastic plate
Mobile Phase: Solvent
Application: Analysis of compounds, checking the purity of samples
Thin layer chromatography is essentially the same as paper chromatography only the stationary phase is changed to a thin layer of silica or alumina on a glass slide. The only advantage of TLC is that the layer can be scraped off the plate for analysis if need be.
The thin layer is applied to a glass slide by preparing a slurry of aluminium oxide in a rapidly drying solvent such as dichloromethane, dipping the slide into the slurry and allowing it to dry.
3. Column Chromatography:
Stationary Phase: Column packed with silica gel or alumina
Mobile Phase: Solvent
Application: Purification of compounds, separation of mixtures
Column chromatography is a larger scale process that allows collection of the components that are separated. The main difference between column and TLC is that the column also uses gravity to draw the mobile phase through the stationary phase. In this case the mobile phase can be continually topped up in the column while the mobile phase (eluent) is run out of the bottom of the column.
Separation and collection of a dissolved mixture.
4. Gas Chromatography (GC):
Stationary Phase: Liquid or polymer on an inert solid support inside a column
Mobile Phase: Inert gas (e.g., helium or nitrogen)
Application: Separation and analysis of volatile compounds
5. High-Performance Liquid Chromatography (HPLC):
Stationary Phase: Column packed with small particle silica gel
Mobile Phase: Liquid solvents under high pressure
Application: Analysis and purification of complex mixtures, pharmaceuticals
Applications:
- 1. Chemical Analysis
- 2. Pharmaceuticals
- 3. Biochemistry
- 4. Environmental Testing
- 5. Forensics
Advantages and disadvantages of chromatographic separation
- High precision and accuracy
- Can separate complex mixtures
- Adaptable to different types of substances
Disadvantages:
- Requires specialized equipment
- Can be time-consuming and costly
- Requires expertise to interpret results