Standard level
Bond polarity deals with separation of charge within a covalent bond, creating a dipole.
Syllabus ref: S2.2.5Structure 2.2.5 - Bond polarity results from the difference in electronegativities of the bonded atoms.
- Deduce the polar nature of a covalent bond from electronegativity values.
Guidance
- Bond dipoles can be shown either with partial charges or vectors.
- Electronegativity values are given in the data booklet.
Tools and links
- Structure 2.1 - What properties of ionic compounds might be expected in compounds with polar covalent bonding?
Electronegativity
The concept of electronegativity is a measure of the electron-attracting ability of an element when covalently bonded to another. To understand why atoms should have different powers to attract electrons we have to look at their size (radius) and their nuclear charges.
Atoms have different sizes and also have different numbers of protons in the nucleus.

In the case of fluorine, a bonded pair of electrons can get much closer to the nuclear charge of the fluorine atom and feels a greater attraction, than in the case of carbon. The elements have increasing electronegativity moving left to right across a period. Fluorine has an electronegativity value of 4.0, whereare carbon has an electronegativity of only 2.5.
|
Electronegativities of the non-metals
|
|||||||
|
C
|
2.5
|
N
|
3.0
|
O
|
3.5
|
F
|
4.0
|
|
Si
|
1.8
|
P
|
2.1
|
S
|
2.5
|
Cl
|
3.0
|
|
|
|
|
|
Se
|
2.4
|
Br
|
2.8
|
|
|
|
|
|
|
|
I
|
2.5
|
Induction of charge
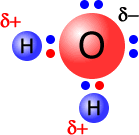
The word 'induction' just means attraction and movement of electrons. The greater the electronegativity of an atom the more it attracts electrons towards itself. Fluorine and oxygen are very good at inducing electrons along bonds towards themselves. The consequence of this is that oxygen and fluorine develop a partial negative charge in comparison to the atoms to which they are bonded.
Partial charges are represented by the Greek letter small delta, followed by the type of charge, positive or negative.
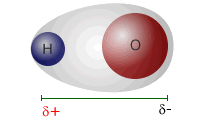
The water molecule, at the left, has an oxygen atom attached by shared pairs of electrons to two hydrogen atoms.
The oxygen is much more electronegative than hydrogen and draws electrons along the bonds towards itself. This results in a partial negative charge on the oxygen and partial positive charges on the hydrogen atoms.
Bond polarity
Logically, when there is induction along a bond by an electronegative element, regions of both positive and negative charge are formed. This is called separation of charge and leads to the concept of bond polarity. The degree of bond polarity can be assessed by considering the difference in electronegativity of the two atoms involved in the bond.
In general, if the bonded atoms have similar electronegativity, then the bond can be considered non-polar, i.e. there is no charge separation.
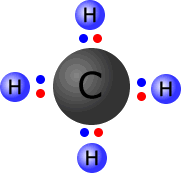 |
Carbon and hydrogen have similar electronegativity, carbon = 2.5 and hydrogen = 2.1. Bonds between carbon and hydrogen are considered to have very little charge separation. We say that the C-H bonds are effectively almost non-polar. |
When a bond has charge separation it is a dipole. Dipoles are vector quantities, i.e. they have both direction and magnitude.
Worked examples
Q231-01 Based on electronegativity values which bond is the most polar?- B-C
- C-O
- N-O
- O-F
|
The greatest difference in electronegativity is between carbon and oxygen (2.5 and 3.5), hence this bond is the most polar. |
Q231-02 Which of the following bonds will have the greatest polarity?
- C-H
- C-N
- C-O
- C-C
|
The largest dipoles occur when there is the greatest difference in electronegativity between atoms. In this case, carbon (electronegativity = 2.5) and oxygen (electronegativity = 3.5) have the greatest difference in electronegativity. Answer = C-O |
Q231-03 Which combination of atoms can form a polar covalent bond?
- H and H
- H and Br
- N and N
- Na and Br
|
Polar covalent bonds form when there is a significant difference in electronegativity between atoms that are bonded together. In this question there is a significant difference in electronegativity between H (2.1) and Br (2.8). There also is a large difference between Na and Br but in this case they form an ionic bond. Therefore the answer is H and Br |