Standard level
A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Effectively the whole unit is one molecule.
Syllabus ref: S2.2.7Structure 2.2.7 - Carbon and silicon form covalent network structures.
- Describe the structures and explain the properties of silicon, silicon dioxide and carbon’s allotropes: diamond, graphite, fullerenes and graphene.
Guidance
- Allotropes of the same element have different bonding and structural patterns, and so have different chemical and physical properties.
Tools and links
- Structure 3.1 - Why are silicon–silicon bonds generally weaker than carbon–carbon bonds?
Allotropes
Allotropes are different physical forms of the same element. They have different structures on a molecular scale.
Allotropic forms can arise in two fundamental ways.
- The atoms themselves can be bonded together in a different way to make different molecules.
- The molecules can pack together in different arrangements
Most elements have allotropic forms:
1 Oxygen can exist in the gaseous state as simple molecules of O2, or ozone, O3.
2 Sulfur can exist in several physical forms, each of which has a different crystal structure.
3 Phosphorus exists as white phosphorus, red phosphorus and purple, or black phosphorus.
However, it is probably carbon that has the most famous allotropes, diamond, graphite, fullerene and graphene. The allotropes are covalent structures with each carbon atom bonded to either three or four other carbon atoms.
Giant molecular structures (macromolecules)
These may be either elements or compounds.
|
Note: Students often ask "but how does the structure end?" The answer is that the final bonding electrons are used up bonding to oxygen, or another suitable element. However, this is unimportant compared to the vast bulk of the atoms on the 'inside' of the structure |
A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Effectively the whole unit is one molecule. Diamond is an example of a three dimensional network solid element.
It clearly is not possible to draw a diagram showing the whole molecular structure of a macromolecular solid, so normally only a representative part is shown.
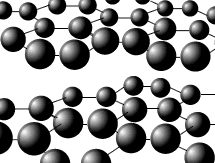
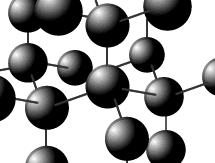 Diamond
is a three dimensional lattice (network) of tetrahedral carbon atoms. The
structure is horribly difficult to draw well, but a small part may be shown
fairly effectively.
Diamond
is a three dimensional lattice (network) of tetrahedral carbon atoms. The
structure is horribly difficult to draw well, but a small part may be shown
fairly effectively.
All of the carbon atoms are bonded to four other carbon atoms in a tetrahedral arrangement. All of the valence electrons are used for bonding. There are no valence electrons free for conduction of electricity. This rigid lattice gives diamond its unique properties. It has high melting and boiling points and is the hardest naturally occurring substance.
Each carbon atom has sp3 Hybridization with bond angles of 109.5º (HL only).
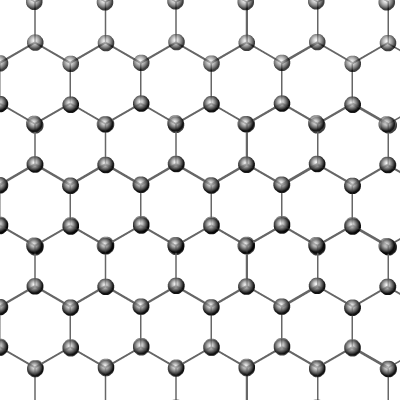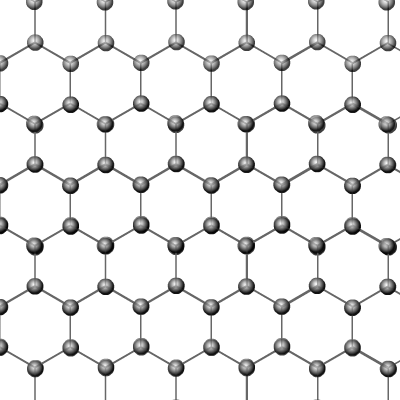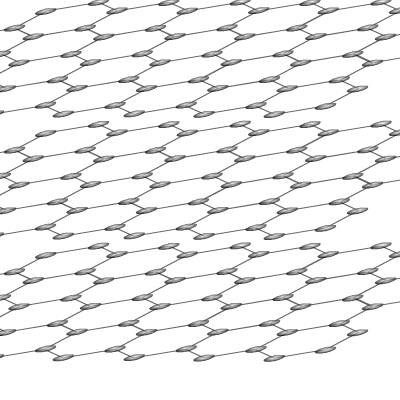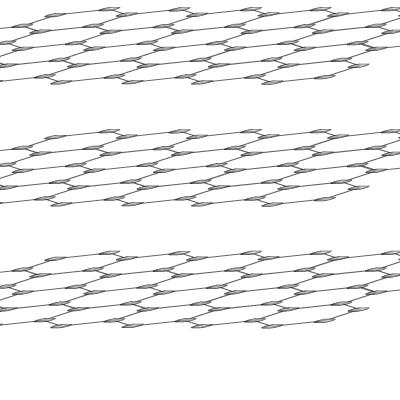
 Graphite
is another naturally occurring allotrope of carbon. In this case the structure
is made up of giant 2-dimensional layers, each layer held together in place
by Van der Waal's attractions.
Graphite
is another naturally occurring allotrope of carbon. In this case the structure
is made up of giant 2-dimensional layers, each layer held together in place
by Van der Waal's attractions.
The layers themselves consist of hexagons of carbon atoms in which each carbon is bonded to three others. This leaves one free electron per carbon atom that can be delocalised throughout a layer and conduct electricity.
Graphite is a black slippery solid with a very high melting and boiling point. It's slippery nature comes from the ability of the layers to slide over one another. This is because the dispersion forces that hold the layers to one another are weak.
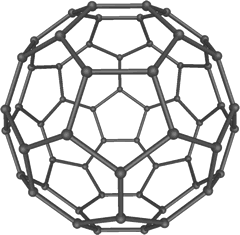 The
graphite layer structure can be folded and bent into balls and tubes providing
the number of carbon atoms in the sheet is large enough. These balls and tubes
(bucky balls and nano tubes) have all been developed from the discovery by
Kroto of spherical molecules consisting of 60 carbon atoms (and greater).
These molecules were called fullerenes after the architect who popularised
the geodhesic dome (Richard Buckminster Fuller), as the sphere is effectively
two hemispherical geodhesic domes stuck together to make a sphere.
The
graphite layer structure can be folded and bent into balls and tubes providing
the number of carbon atoms in the sheet is large enough. These balls and tubes
(bucky balls and nano tubes) have all been developed from the discovery by
Kroto of spherical molecules consisting of 60 carbon atoms (and greater).
These molecules were called fullerenes after the architect who popularised
the geodhesic dome (Richard Buckminster Fuller), as the sphere is effectively
two hemispherical geodhesic domes stuck together to make a sphere.
Fullerene is an allotrope of carbon with 60 carbon atoms in the molecule. It is not a giant structure (C60 is just not that large when compared to 'real' giant structures, such as graphite and diamond). It was first isolated from soot and can be crystallised as black, or red crystals, with a melting point of about 280ºC. Fullerene is soluble in many non-polar solvents.
The structure consists of five and six membered carbon rings in which each
carbon atoms is attached to three other carbon atoms. The Hybridization at
each carbon is sp2 (HL only). It has delocalised electrons spread
out over the whole structure in one large molecular orbital.
Graphene is a newly discovered allotrope of carbon which effectively consists of single layers of graphite. It has many potentially exciting properties and there are currently many research facilities trying to develop new ways to prepare the graphene sheets rapidly and in a pure form.
Graphene is about 200 times stronger than steel and nearly transparent. It is the worlds most conducting material and is 1,000,000 times thinner than a human hair. ref
It has been suggested that new graphene technology could produce transparent and flexible computers.
Silicon also forms giant macromolecular structures similar to diamond, in which all of the valence electrons are used to link each of the silicon atoms into a giant array of tetrahedral atoms.
The properties of silicon is consistent with its macromolecular structure. It is a hard solid with a very high melting and boiling point.
Silicon dioxide is a macromolecular compound with a structure which can be thought of as having oxygen atoms interposed between each pair of silicon atoms within the diamond-type lattice.
Silicon dioxide has the characteristic properties of a macromolecular compound; a high melting and boiling point and very hard. All of its valence electrons are used in bonding so it is a non-conductor. The silicon atoms (and the oxygen atoms) use sp3 Hybridization.
- I - The poorest electrical conductor of the three is diamond
- II - The atoms in graphite and C60 fullerene are attached to three other carbon atoms
- II - The atoms in diamond and C60 fullerene are arranged in hexagons
- I and II only
- I and III only
- II and III only
- I, II and III
Correct response - A options I and II only |
Q214-02 State the structures of, and the bonding, in diamond and graphite.
Answer
|
Diamond and graphite are both giant molecular structures. In diamond, each carbon is bonded to four others, and in graphite each carbon atoms is bonded to only three others in layers. Each layer in graphite has an associated delocalised electron layer and is held to other layers by weak dispersion forces. |
Q214-03 Compare and explain the hardness and electrical conductivity of diamond and graphite.
Answer
|
Diamond is a hard, rigid structure, as each carbon atom is held in place by four strong covalent bonds. It is a non-conductor, as all of its valence electrons are used in bonding. Graphite is a soft slippery solid, as the layers can slide easily over one another, because they are held only by weak dispersion forces. The carbon atoms use only three out of four electrons to make the framework structure of a layer and the remaining electron is delocalised over the whole layer, allowing graphite to conduct well. |
Q214-04 Predict and explain how the hardness and electrical conductivity of C60 fullerene would compare with that of diamond and graphite.
Answer
|
Although there is delocalisation within a C60 fullerene molecule, it is expected to be a non conductor as the simple molecules are not connected to one another by any formal bonds. It is similarly expected to be soft. Diamond is a hard, rigid structure, as each carbon atom is held in place by four strong covalent bonds. It is a non-conductor, as all of its valence electrons are used in bonding. Graphite is a soft slippery solid, as the layers can slide easily over one another, because they are held only by weak dispersion forces. The carbon atoms use only three out of four electrons to make the framework structure of a layer and the remaining electron is delocalised over the whole layer, allowing graphite to conduct well. |