Standard level
Syllabus ref: S2.4.1Structure 2.4.1 - Bonding is best described as a continuum between the ionic, covalent and metallic models, and can be represented by a bonding triangle.
- Use bonding models to explain the properties of a material.
Guidance
- A triangular bonding diagram is provided in the data booklet.
Tools and links
- Structure 3.1 - How do the trends in properties of period 3 oxides reflect the trend in their bonding?
- Nature of science, Structures 2.1, 2.2 - What are the limitations of discrete bonding categories?
Classification of bonding
Nowadays there are many different types of materials in everyday use in society and industry, all of which have been specifically designed and manufactured for their intended purpose.
We have come a long way from stone and wooden tools
An understanding of the underlying structure of the materials and its consequences in terms of property has been essential in this process.
From the previous sections on ionic, covalent and metallic bonding we have seen how the structure influences the physical property of the material.
However, the concepts of bonding can get blurred around the edges, as covalent compounds can exhibit a degree of ionic behaviour in terms of polarity, while ionic compounds can exhibit a degree of covalent character. We see that the classification of matter becomes more of a gradual change from one bonding type to another with a commensurate gradual change of property.
This gives rise to the term "bonding continuum", where boundaries between the major structural types are not well defined.
Napoleon's army and the Russian campaign of 1812
There is a theory that degradation of the properties of tin due to the cold, contributed to the defeat of Napoleon's army in the winter of 1812.
The story goes that the army uniforms were held together by buttons made of tin. Under normal European conditions this was just fine and the buttons did their job.
However, in the harsh Russian winter the low temperatures caused the tin to change from the metal allotrope to a non-metal allotrope (alpha tin), with disasterous consequences on its properties.
The buttons lost their hardness and became soft and crumbly. They fell apart, as did the soldiers' uniforms.
While this may just be an apocryphal "urban legend", it highlights the link between structure and property in terms of materials.
The van Arkel Ketelaar bonding triangle
The bonding triangle shows us how to predict the type of bonding within a structure by referring to the electronegativities of the bonded elements.
Mixtures and composites
Mixtures
A mixture is a combination of two or more substances that are physically combined but not chemically bonded. Each substance in a mixture retains its own properties.
Mixtures may be:
- 1. Homogeneous
- 2. Heterogeneous
Homogeneous Mixtures are mixtures where the components are evenly distributed, and appear to be the same throughout. Examples include salt water and air.
Heterogeneous Mixtures are mixtures where the components are not evenly distributed, and you can see the different parts. Examples include a salad and a mixture of sand and iron filings.
Properties of Mixtures:
- Components can be separated by physical methods (e.g., filtration, distillation).
- The composition can vary (e.g., you can add more sugar to tea to make it sweeter).
Uses of Mixtures:
- **Air:** Essential for breathing, contains oxygen, nitrogen, and other gases.
- **Salt Water:** Used in cooking and preserving food.
- **Alloys:** Mixtures of metals, like steel (iron and carbon), are used in construction and manufacturing.
Composites
A composite is a material made from two or more different substances that, when combined, produce a material with characteristics different from the individual components. They are engineered to have better properties.
Examples of Composites:
- 1. Fiberglass
- 2. Carbon Fibre
Fibreglass is made from plastic reinforced with glass fibers. It is strong and lightweight. Carbon Fibre is made from carbon filaments (fibres) reinforced with plastic. It is very strong and light, often used in sports equipment and aerospace.
Properties of Composites:
- Enhanced strength and durability.
- Often lighter than pure metals.
- Resistant to corrosion and wear.
Uses of Composites:
Composites are used in building materials like concrete reinforced with steel rebar.
Automotive and Aerospace: Used to make lightweight and strong parts for cars and airplanes.
Sports Equipment: Used in making bicycles, tennis rackets, and golf clubs for better performance.
In summary, mixtures are combinations of substances that retain their own properties and can be separated physically, while composites are engineered materials that combine substances to produce new properties, making them useful in a wide range of applications.
Worked examples
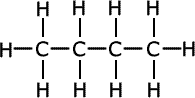
Q271-01 Select the substance with the higher boiling point in each of the following pairs. Explain your reasoning:- C2H6 and C3H8
- CH3CH2OH and CH3OCH3
|
In the first pair of compounds there are no dipoles so the intermolecular forces are due to dispersion attractions only. The larger of the two molecules has the greater intermolecular force. Therefore C3H8 has the higher boiling point. In the second pair of compounds their relative masses are the same, but the alcohol, CH3CH2OH, has hydrogen bonding due to the OH group, whereas the ether (methoxymethane) has only weak dipole - dipole interactions. The alcohol, CH3CH2OH, has the higher boiling point. |
Q271-02 Which substance has the highest boiling point?
- CH4
- He
- HF
- Cl2
|
Q271-03 The very high heat of vaporization of H2O is mainly a result of
- dispersion forces
- covalent bonds
- inter-ionic attractions
- hydrogen bonding
|
The enthalpy of vaporisation is the amount of energy needed to turn one mole of a substance from liquid into vapour. It is a measure of the strength of the intermolecular forces. Water's high enthalpy of vaporisation is due to the strengthof intermolecular forces caused by hydrogen bonding. |
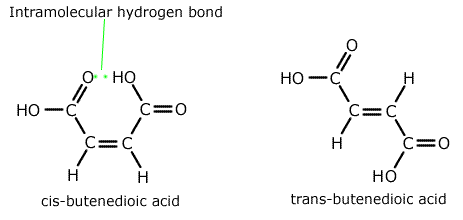
Q271-04 Explain the following statement: "Cis-butenedioic acid has a lower melting point than its isomer trans-butenedioic acid."
Answer
|
The lower melting point of the cis-butenedioic acid is indicative of the weaker intermolecular forces compared to the trans isomer. To understand why it is necessary to look at the structure of each isomer.
|
Q271-05 Arrange the following compounds in order of increasing boiling point and explain your choice by reference to the intermolecular forces involved: Bromoethene, chloroethene, ethene
Answer
|
Ethene, CH2=CH2, is a non-polar simple molecular substance with a low boiling point. However, both bromoethene, CH2=CHBr and chloroethene, CH2=CHCl are polar substances with similar polarity. Bromoethene has a much larger relative mass than chloroethene and so larger dispersion forces. This is the deciding factor, leaving the order of increasing boiling point as ethene < chloroethene < bromoethene. |
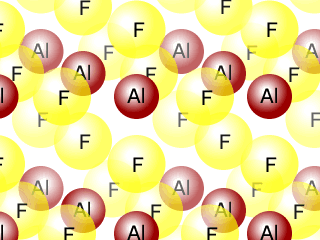
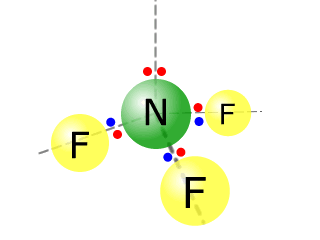
Q271-06 Aluminium fluoride AlF3 is a solid up to temperatures of 1250ºC, whereas nitrogen trifluoride is a gas above -129ºC. Describe the bonding and structure in samples of each of these substances.
Answer
|
Aluminium fluoride has a giant ionic structure, whereas nitrogen trifluoride has a simple molecular structure.
|
Q271-07 Which statements are generally true about the melting points of substances?
- I - Melting points are higher for compounds containing ions than for compounds containing molecules.
- II - A compound with a low melting point is less volatile than a compound with a higher melting point.
- III - The melting point of a compound is decreases by the presence of impurities.
- I only
- I and III only
- II and III only
- I, II and III
|
I - Melting points are higher for compounds containing ions than
for compounds containing molecules - true Correct response I and III only |
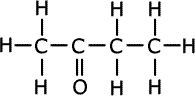
Q271-08 CH3COCH3 is the first member of the ketone homologous series. Draw the full structural formula of the next member of the homologous series and predict how its melting point compares with that of CH3COCH3
Answer
|
Members of homologous series differ by one -CH2- unit. The first member of the ketones (alkanones) is propanone, thus the second member is butanone, CH3COCH2CH3.
|
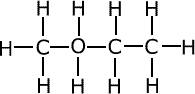
Q271-09 The compounds A, B and C have approximately the same molar mass:
| A | B | C |
| C4H10 | CH3CH2CH2OH | CH3OCH2CH3 |
When the compounds are arranged in order of increasing boiling points (lowest
boiling point first), the correct order is:
- A, C, B
- A, B, C
- B, C, A
- C, B, A
|
The three compounds belong to different homologous series, alkanes, alcohols and ethers. As they have the same relative mass we can discount the effect of dispersion forces. Alkanes are non-polar and have very low melting and boiling points. Ethers are weakly polar and have higher boiling points than alkanes. Alcohols have extensive hydrogen bonding and the highest boiling point of the three groups. The correct order of increasing boiling point is A, C, B.
|
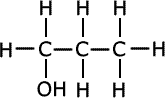
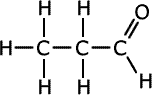
Q271-10 Identify the strongest type of intermolecular force present in each of the compounds propan-1-ol, propanal and propanoic acid. List these compounds in decreasing order of boiling point.
Answer
|
The three compounds belong to different homologous series, alcohols, aldehydes (alkanals) and carboxylic acids (alkanoic acids). Although all three compounds have different relative masses, they are not extremely different and the effect of the functional groups is greater than that of dispersion forces. In propanoic acid there is extensive hydrogen bonding and so it has the highest boiling point. The next highest boiling point is in propan-1-ol which also has hydrogen bonding. The aldehyde has only weaker dipole-dipole interactions.
|